What is PLX3397 (Pexidartinib)?
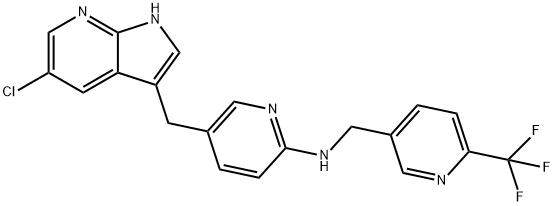
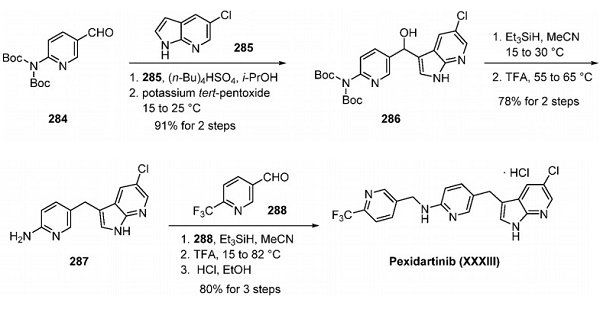
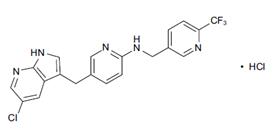
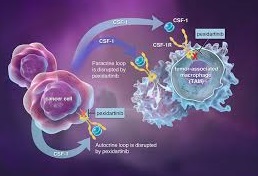
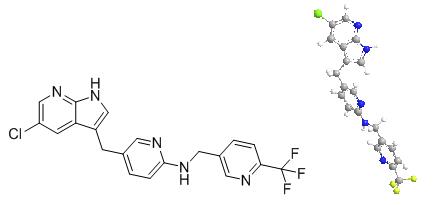
PLX3397 (pexidartinib; N-[5-[(5-chloro-1H-pyrrolo[2,3-b]pyridin-3-yl)methyl]-2-pyridinyl]-6-(trifluoromethyl)-3-pyridinemethanamine, 5-((5-chloro-1H-pyrrolo[2,3-b]pyridin-3-yl)methyl)-N-((6-(trifluoromethyl)pyridin-3-yl)methyl)pyridin-2-amine; N-[5-[(5-Chloro-1H-pyrrolo[2,3-b]pyridin-3-yl)methyl]-2-pyridinyl]-6-(trifluoromethyl)-3-pyridinemethanamine; C20H15ClF3N5) [1] is a small-molecule, oral, potent multi-targeted receptor tyrosine kinase (RTK) inhibitor of CSF-1R, Kit, and Flt3 and it can cross the blood-brain barrier. In M-NFS-60, Bac 1.2F5 and M-07e cells, PLX3397 (Pexidartinib) inhibits the CSF1-dependent proliferation with IC50 of 0.44 μM, 0.22 μM and 0.1 μM, respectively [2]. It has been reported that PLX3397 strongly attenuates systemic and local accumulation of macrophages driven by B16F10 melanoma and does not affect Gr-1+ bone marrow-derived suppressor cells. Besides, PLX3397 weakly inhibited the growth of SK-N-SH cells with an IC50 of 10 μM and had little effect on the growth of MDA-MB-231 human tumor cell xenografts [5]. In MMTV-PyMT mice, pexidartinib (40 mg/kg, p.o.) significantly inhibits both steady-state and PTX-induced tumor infiltration by CD45 + CD11b + Ly6C − Ly6G − F4/80 +. Pexidartinib/PTX therapy also results in a significant reduction in CD31+ vessel density within mammary tumors, paralleling induction of apoptosis and necrosis [2]. In C57 mice bearing GL261 tumors, Pexidartinib (p.o.) inhibits glioblastoma invasion [3]. In CMO mice, PLX3397 significantly attenuates autoinflammatory disease by decreasing the erosive bone lesions in tails and paws and the levels of circulating MIP-1α [4]. In mice bearing B16F10 melanomas, Pexidartinib (45 mg/kg, p.o.) enhances CD8-mediated immunotherapy of melanoma [5].
Administration of PLX3397 reduced CIBP, induced substantial intratumoral fibrosis, and was also highly efficacious in reducing tumor cell growth, formation of new tumor colonies in bone, and pathological tumor-induced bone remodeling. PLX3397 is superior to imatinib in the treatment of malignant peripheral nerve sheath tumor (MPNST), and the combination of PLX3397 with a TORC1 inhibitor could provide a new therapeutic approach for the treatment of this disease [6].
Plexxikon Inc. (USA), a subsidiary of Daiichi Sankyo, is developing pexidartinib for treating cancer. Plexxikon is conducting phase III clinical studies with PLX-3397 for the treatment of pigmented villonodular synovitis. Phase II clinical studies are ongoing for the oral treatment of melanoma and glioblastoma multiforme. No recent development has been reported from preclinical studies for the treatment of systemic lupus erythematosus and for the treatment of multiple sclerosis. Prior to patient enrollment, a phase I clinical trial by Plexxikon for the treatment of rheumatoid arthritis was withdrawn. Daiichi Sankyo (parent of Plexxikon) decided to discontinue phase II trials of the product for the treatment of castration-resistant prostate cancer and for the treatment of Hodgkin’s lymphoma after reviewing its clinical study results and also have discontinued phase II studies for the treatment of acute myeloid leukemia due to strategic reasons [6].
Recently, some scholars have found that PLX3397 can effectively treat advanced giant cell tumor of tendon sheath. Giant cell tumor of the tendon sheath (TGCT) is a rare locally invasive tumor that overexpresses colony stimulating factor 1 (CSF1). Surgery is the standard of care, and there are currently no approved systemic treatment options. Researchers try to evaluate that the possibility of PLX3397 (Pexidartinib) as a CSF1 receptor inhibitor as a viable systemic treatment option in TGCT patients, especially if these patients are not suitable for surgical resection. The results were gratifying. 120 patients with advanced TGCT who were inoperable were randomized to receive a total effective rate of 39% in the PLX3397 (Pexidartinib) group after week 25, which was significantly higher than the placebo group[7].
References
[1] http://drugapprovalsint.com/pexidartinib-plx-3397/
[2] Denardo D G , Brennan D J , Rexhepaj E , et al. Leukocyte Complexity Predicts Breast Cancer Survival and Functionally Regulates Response to Chemotherapy [J]. Cancer Discovery, 2011, 1(1):54-67.
[3] Coniglio S J , Eugenin E , Dobrenis K , et al. Microglial Stimulation of Glioblastoma Invasion Involves Epidermal Growth Factor Receptor (EGFR) and Colony Stimulating Factor 1 Receptor (CSF-1R) Signaling[J]. Molecular Medicine, 2012, 18(1):519-527.
[4] Chitu V , Nacu V , Charles J F , et al. PSTPIP2 deficiency in mice causes osteopenia and increased differentiation of multipotent myeloid precursors into osteoclasts[J]. Blood, 2012, 120(15):3126-3135.
[5] Sluijter M , Sluis T C V D , Velden P A V D , et al. Inhibition of CSF-1R Supports T-Cell Mediated Melanoma Therapy[J]. PLOS ONE, 2014, 9(8):e104230.
[6] https://www.medchemexpress.com/Pexidartinib.html?src=google-product&gclid=EAIaIQobChMIyMnv5M2x5QIVFXZgCh2guwZJEAAYASAAEgLbV_D_BwE
[7] https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(19)30764-0/fulltext
You may like
Related articles And Qustion
Lastest Price from PLX3397 (Pexidartinib) manufacturers
US $1.10/g2021-08-18
- CAS:
- 1029044-16-3
- Min. Order:
- 1g
- Purity:
- 99.9%
- Supply Ability:
- 100 Tons min

US $15.00-10.00/KG2021-07-13
- CAS:
- 1029044-16-3
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton