What is Omidenepag Isopropyl
Description
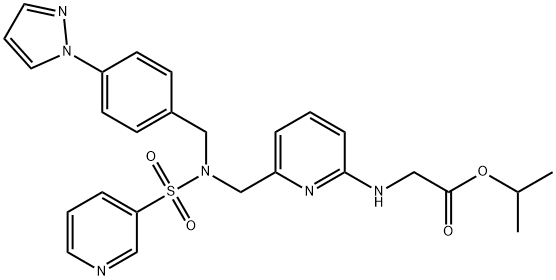
Omidenepag isopropyl (OMDI), administered as a 0.002% ophthalmic solution, was approved in Japan in 2018 for treating glaucoma and ocular hypertension. Discovered and developed by Santen, the drug was designed as an isopropyl ester to allow for increased passive permeability to the corneal epithelium, where the physiologic pH of this compartment would convert the molecule to the corresponding carboxylate, revealing a potent and selective prostaglandin E2 (h-EP2) receptor agonist.
Biological activity
OMDI is a prostanoid receptor agonist that is selective for the EP2 receptor. It affects the uveoscleral and trabecular outflow, thereby facilitating aqueous humor outflow. The EP2 receptor is in the ciliary body, trabecula, iris, cornea, conjunctiva, and retina. The IOP decreases through smooth muscle relaxation and the effects on the extracellular matrix[1].
Synthesis method
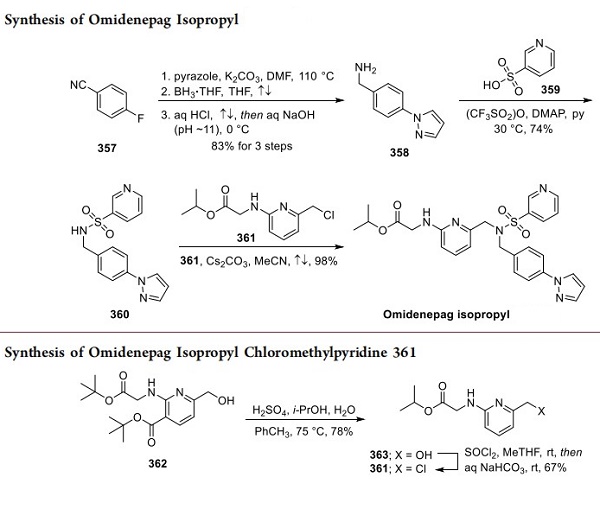
Two patent applications disclosed by the Japanese firm Ube Industries, Ltd. have described a process synthesis of omidenepag isopropyl. Reacting p-cyanofluorobenzene (357) with pyrazole in the presence of base, borane reduction of the nitrile, and acid−base workup led to 358 in 83% across the three-step sequence, shown above[2]. Next, the subjection of sulfonic acid 359 to triflic anhydride prior to exposure to 358 under basic conditions yielded sulfonamine 360. Lastly, reaction with 361 (arising from exposure of compound 362 in above to sulfonic acid in warm aqueous isopropanol, giving 363 and subsequent treatment with thionyl chloride and aqueous base) under basic conditions in refluxing acetonitrile furnished omidenepag isopropyl in an impressive 98% yield. It should be noted that although tert-butyl nicotinate 362 is commercially available, the source of this starting material was not specified in the Ube patent.
References
[1] Kenji Inoue . “Periocular Adverse Reactions to Omidenepag Isopropyl.” American Journal of Ophthalmology 237 (2022): Pages 114-121.
[2] Andrew C. Flick. “Synthetic Approaches to New Drugs Approved during 2018.” Journal of Medicinal Chemistry 63 19 (2020): 10652–10704.