What is Mepivacaine?
Brief Introduction
Mepivacaine hydrochloride is available in 1% to 3% solutionsand is indicated for infiltration anesthesia, dental procedures,peripheral nerve block, or epidural block. The onset of anesthesiais rapid, ranging from about 3 to 20 minutes for sensoryblock. Mepivacaine is rapidly metabolized in the liver with50% of the administered dose excreted into the bile asmetabolites. The metabolites are reabsorbed in the intestineand excreted in the kidney with only a small percentage foundin the feces. Less than 5% to 10% of the administered dose isfound unchanged in the urine. The primary metabolic productsare the N-demethylated metabolite and the 3 and 4 phenolicmetabolites excreted as their glucuronide conjugates.
Potency
Pharmacologically like lidocaine, mepivacaine is a xylidine derivative. Mepivacaine is similar to lidocaine in its onset of action, duration, potency, toxicity, and no reported allergic reactions. Mepivacaine is available in two different formulations: 3% mepivacaine plain, and 2% mepivacaine 1:20,000 levonordefrin. Because mepivacaine produces less vasodilation than lidocaine, it is an effective anesthetic without a vasoconstrictor and is supplied in this manner only in the 3% formulation. It can be used for short appointments providing pulpal anesthesia of approximately 20 minutes via supraperiosteal injections, and 40 minutes via nerve blocks, and 2 to 3 hours of soft tissue anesthesia when profound pulpal anesthesia is not necessary. It is therefore a good alternative if the use of a vasoconstrictor is contraindicated. However, caution should be taken to avoid systemic toxicity related to using plain anesthetics, especially in children. Two percent mepivacaine is the only anesthetic that is formulated with levonordefrin as its vasoconstrictor in the United States, and it provides equivalent depth and duration of pulpal (60 minutes) and soft tissue (3 to 5 hours) anesthesia as lidocaine with epinephrine. Levonordefrin, however, does not provide the same intensity of hemostasis as epinephrine. Similar to lidocaine, mepivacaine also has anticonvulsant properties.
Mepivacaine is equal in potency to lidocaine and prilocaine, and it is two-thirds as potent as articaine and one-fourth as potent as bupivacaine. Mepivacaine is similar in toxicity to lidocaine and articaine (about equal or slightly less [25%]). It is more toxic than prilocaine (approximately 25% more). It is far less toxic than bupivacaine, only one-quarter as toxic. Mepivacaine is not effective as a topical anesthetic. Like lidocaine, mepivacaine is metabolized in the liver using several hepatic enzymes, and variable excretion of unchanged mepivacaine from zero to 16% by the kidneys. Individuals with significant hepatic disease can receive mepivacaine but at a reduced dose.
The maximum recommended dose for mepivacaine is 3.0 mg/lb or 6.6 mg/kg, and the absolute MRD is 400 mg (see Table 5-14 for mepivacaine MRDs and Chapter 8 for calculation guidelines).
Clinical Use
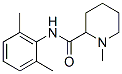
Mepivacaine hydrochloride [N-(2, 6-dimethylphenyl)-1-methyl 2-piperidinecarboxamide monohydrochloride] is an amino amide-type local anesthetic agent widely used to provide regional analgesia and anesthesia by local infiltration, peripheral nerve block, and epidural and caudal blocks. The pharmacological and toxicological profile of mepivacaine is quite similar to that of lidocaine, except that mepivacaine has a slightly longer duration of action and lacks the vasodilator activity of lidocaine. For this reason, it serves as an alternate choice for lidocaine when addition of epinephrine is not recommended in patients with hypertensive vascular disease.
Metabolism
Mepivacaine undergoes extensive hepatic metabolism catalyzed by CYP1A2, with only a small percentage of the administered dosage (<10%) being excreted unchanged in the urine. The major metabolic biotransformations of mepivacaine are N-dealkylation (to give the N-demethylated compound 2′,6′-pipecoloxylidide) and aromatic hydroxylations. These metabolites are excreted as their corresponding glucuronides.
Manufacturing Process
Ethyl magnesium bromide is prepared in the usual way by reacting 185 parts by weight of ethyl bromide in 800 parts of anhydrous ether with 37 parts by weight of magnesium turnings. Under vigorous stirring 121 parts of 2,6- dimethyl aniline are added at a rate depending on the vigor of the gas evaporation. When the evolution of gas has ceased, 85 parts by weight of Nmethylpipecolic acid ethyl ester are added to the 2,6-dimethyl aniline magnesium bromide slurry. The mixture is refluxed for ? hour with continued stirring, after which it is cooled down. Dilute hydrochloric acid is added carefully in order to dissolve and hydrolyze the magnesium compound formed.
The pH is adjusted to 5.5 and the water phase separated and extracted with additional ether in order to remove the surplus dimethyl aniline. After addition of an excess of ammonia to the solution, the reaction product, Nmethylpipecolic acid 2,6-dimethyl anilide, is recovered by extraction with isoamyl alcohol. The isoamyl alcohol solution is evaporated to dryness, the product dissolved in dilute hydrochloric acid, treated with charcoal and reprecipitated with NaOH. N-methylpipecolic acid 2,6-dimethyl anilide is obtained in crystalline form.
You may like
Related articles And Qustion
See also
Lastest Price from Mepivacaine manufacturers

US $0.00/KG2025-04-22
- CAS:
- 22801-44-1
- Min. Order:
- 1KG
- Purity:
- 0.99
- Supply Ability:
- 1000KG

US $0.00-0.00/kg2025-04-21
- CAS:
- 22801-44-1
- Min. Order:
- 25kg
- Purity:
- 0.99
- Supply Ability:
- 1T