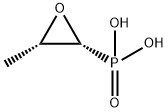
What is Fosfomycin?
Fosfomycin is a phosphoenolpyruvate analogue produced by Streptomyces fradiae and by some Pseudomonas spp., but is now mostly obtained synthetically. It is a broad-spectrum antibiotic with bactericidal properties. Fosfomycin’s mechanism of action is bacterial cell wall inhibition by binding to and inactivating the enzyme enolpyruvate transferase. This leads to an irreversible blockage of the condensation of uridine diphosphate-N-acetylglucosamine with p-enolpyruvate, which is one of the first steps of bacterial cell wall synthesis, thereby eventually causing cell lysis and bacterial cell death.
Fosfomycin can be administered intravenously as disodium fosfomycin and is also available as a calcium or trometamol (synonym: tromethamin) salt that can (both) be administered orally. Fosfomycin has a broad spectrum of activity against aerobic bacteria including those commonly responsible for urinary tract infection (except most strains of P. aeruginosa) and is mostly used for that indication. It is the only representative of its drug class and with its target site of action unaffected by other antibiotics there is consequently no cross-resistance with other antibiotics.
Mechanism of Action
Fosfomycin is a cell wall inhibitor. Growth and survival of bacteria rely on the functionality of the enzyme MurA (UDP-N-acetylglucosamine enolpyruvyl transferase). This enzyme catalyzes the first step in the biosynthesis of the bacterial cell wall. MurA is the target of fosfomycin, which covalently attaches to the thiol group of a cysteine in the active site of E. coli MurA and thus irreversibly inhibits the enzymatic function. Mutation of the cysteine to asparagine renders Chlamydia spp., among others, resistant to fosfomycin.
At least in E. coli, fosfomycin uses two uptake systems as a means of entry: the L-alpha-glycerophosphate and the hexose-6-phosphate transport system (GlpT and UhpT transporters). Expression of these genes requires the presence of the cyclic AMP–cyclic AMP receptor protein complex. Defects in one or both of the transport systems, either structural genes or regulator genes, will result in resistance to fosfomycin. Glucose 6-phosphate induces the hexose phosphate transport system. Maximal enhancement of fosfomycin activity is found with 25 mg/ml G-6-P, which is now usually recommended for susceptibility testing of fosfomycin.
Clinical uses
Fosfomycin is being used for a limited number of indications. The main clinical use of fosfomycin is for the treatment of uncomplicated UTI, but it also appears to be effective for recurrent UTI. There are sporadic reports of smaller trials of fosfomycin alone or in combination with other antibiotics for the treatment of surgical infections and for use in surgical prophylaxis.
You may like
Lastest Price from Fosfomycin manufacturers

US $0.00/kg2025-04-25
- CAS:
- 23155-02-4
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg

US $0.00-0.00/KG2025-04-15
- CAS:
- 23155-02-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg