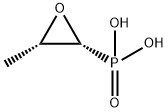
Fosfomycin
- Product NameFosfomycin
- CAS23155-02-4
- CBNumberCB7446605
- MFC3H7O4P
- MW138.06
- EINECS245-463-1
- MDL NumberMFCD00242804
- MOL File23155-02-4.mol
Chemical Properties
| Melting point | 94°C |
| Boiling point | 342.7±52.0 °C(Predicted) |
| Density | 1.56±0.1 g/cm3(Predicted) |
| pka | 3.20±0.40(Predicted) |
| form | solid |
| CAS DataBase Reference | 23155-02-4(CAS DataBase Reference) |
| FDA UNII | 2N81MY12TE |
| ATC code | J01XX01 |
Fosfomycin Chemical Properties,Usage,Production
Mode of action
The N-acetylmuramic acid component of the bacterial cell wall is derived from N-acetylglucosamine by the addition of a lactic acid substituent derived from phosphoenolpyruvate. Fosfomycin blocks this reaction by inhibiting the pyruvyl transferase enzyme involved. The antibiotic enters bacteria by utilizing active transport mechanisms for α-glycerophosphate and glucose-6-phosphate. Glucose-6-phosphate induces the hexose phosphate transport pathway in some organisms (notably Escherichia coli) and potentiates the activity of fosfomycin against these bacteria.Description
Fosfomycin is unique in possessing a simple epoxide ring and has a broad activity spectrum against gram-positive and gramnegative bacteria .Chemical Properties
Water-soluble crystals.Originator
Fosfocin,Crinos,Italy,1977Uses
Antibacterial.Definition
ChEBI: A phosphonic acid having an (R,S)-1,2-epoxypropyl group attached to phosphorus.Manufacturing Process
(A) The preparation of [(1-chloroethoxy)chloromethyl]phosphonic acid: Acetaldehyde (1.1 mol) and hydroxymethylphosphonic acid (1 mol) in 500 ml of benzene are saturated with hydrogen chloride gas at 10°C to 15°C. The mixture is aged at 25°C for 24 hr, the solvent distilled out in vacuo and the residue flushed three times with benzene to remove all traces of hydrogen chloride. The residue is taken up in benzene (500 ml), treated with tert-butyl hypochlorite (0.8 mol) and azobisisobutyronitrile (0.8 mm) at 40°C until titration shows the absence of hypochlorite and the solution is then evaporated to yield [(1-chloroethoxy)chloromethyl] phosphonic acid in the form of an oil.(B) The preparation of (cis-1,2-epoxypropyl)phosphonic acid: [(1- chloroethoxy)chloromethyl] phosphonic acid (1.0 g) is added with stirring to tetrahydrofuran (50 ml) to which has been added a crystal of iodine and a zinc-copper couple (15.0 g). The mixture is then heated under reflux for 24 hr and the resulting solution filtered to yield (cis-1,2-epoxypropyl)-phosphonic acid.
There is also a fermentation route to Fosfomycin as noted by Kleeman and Engel.
Therapeutic Function
AntibioticBiological Activity
Fosfomycin shows antibacterial activity against gram-positive and gram-negative organisms, including Pseudomonas aeruginosa andSerratiamarcescens,andβ-lactam-resistant Staphylococcus aureus. Its mechanism of action is probably the inhibition of cell-wall synthesis. It shows no cross-resistance with other classes of antibiotics.Biological Activity
Fosfomycin shows antibacterial ac tivity against gram-positive and gram-negative organisms, including Pseudomonas aeruginosa andSerratiamarcescens,andβ-lactam-resistant Staphylococcus aureus. Its mechanism of action is probably the inhibition of cell-wall synthesis. It shows no cross-resistance with other classes of antibiotics.Clinical Use
Phosphomycin, introduced in 1972, inhibits enolpyruvial transferase, an enzyme catalyzing an early step in bacterial cell wall biosynthesis. Inhibition results in reduced synthesis of peptidoglycan, an important component in the bacterial cell wall. Phosphomycin is bactericidal against Escherichi a coli and Enterobacter faecali s infections.Drug interactions
Potentially hazardous interactions with other drugsMetoclopramide: increases gastrointestinal motility and therefore lowers the serum concentration and urinary excretion of fosfomycin.
Metabolism
Fosfomycin undergoes no biotransformation and is excreted mainly unchanged through the kidneys. This results in very high urinary concentrations (up to 3 mg/mL) within 2-4 hours of a dose. Therapeutic concentrations of 200-300 mcg/mL in urine are usually maintained for at least 36 hours, and can last from 48-72 hours.structure and hydrogen bonding
Fosfomycin's chemical structure is simple anduniqueamongantibiotics inhavinga C–P bond.Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
Fosfomycin Supplier
Global(116)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| +8617531153977 | allison@yan-xi.com | China | 5855 | 58 | |
| +8615531151365 | mina@chuanghaibio.com | China | 18137 | 58 | |
| +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21631 | 55 | |
| +86-0371-86658258 +8613203830695 |
factory@coreychem.com | China | 29808 | 58 | |
| +1-781-999-5354 +1-00000000000 |
marketing@targetmol.com | United States | 32159 | 58 | |
| +86-0551-65418671 +8618949823763 |
sales@tnjchem.com | China | 34563 | 58 | |
| +86-029-89586680 +86-18192503167 |
1026@dideu.com | China | 7732 | 58 | |
| +86-0571-85134551 | sales@afinechem.com | China | 15350 | 58 | |
| +86-057181025280; +8617767106207 |
sales@molcore.com | China | 49734 | 58 | |
| +8618058761490 | info@gihichemicals.com | China | 49984 | 58 |
Related articles
Fosfomycin is a phosphoenolpyruvate analogue produced by Streptomyces fradiae and by some Pseudomonas spp., but is now mostly obtained synthetically. It is a broad-spectrum antibiotic with bactericidal properties.
Mar 18,2022
23155-02-4, FosfomycinRelated Search
- Zoledronic acid
- 1-Hydroxyethylidene-1,1-diphosphonic acid
- Citric acid
- Folic acid
- Disodium pamidronate
- Phosphorous acid
- Ethyl 2-(Chlorosulfonyl)acetate
- Amino tris(methylene phosphonic acid)
- Ascoric Acid
- Fosfomycin sodium
1of4
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine


