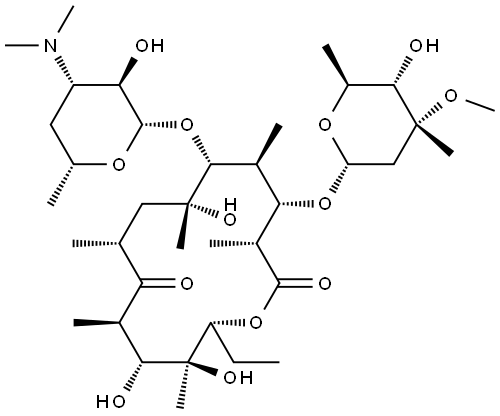
What is Erythromycin?
Erythromycin (CAS number: 114-07-8), thereafter renamed erythromycin A, was first isolated at Eli Lilly from a strain of Streptomyces erythreus. It is the first representative of the class of macrolide antibiotics introduced for clinical use. Macrolides are characterized by a macrocyclic lactone ring substituted by two sugars, among which a desosamine confers a character of weak base responsible for their ability to accumulate inside the cells. Erythromycin A base is very bitter, insoluble in water, and inactivated by acid (including gastric secretions) as a result of an intramolecular cyclization reaction leading to the formation of an inactive spirocetal compound.
Effort has thus been made to develop gastro-resistant formulations or ester prodrugs to improve oral absorption. Current formulations of erythromycin base include different formulations to be administered by the oral route and a powder to be reconstituted for intravenous administration. The availability of these differs from one country to another (tablets, gastro-resistant capsules with delayed release, and granules or powder for reconstitution of oral solutions). Erythromycin is also an active ingredient in several preparations for topical applications. Stearate, estolate, ethylsuccinate, lactobionate, and gluceptate are the salt and ester forms used for clinical use. Most of the oral formulations need to be administered 1 hour before a meal to ensure optimal oral absorption because food affects drug absorption.
There are four key erythromycin compounds that have been used clinically:
1. Erythromycin stearate (a salt).
2. Erythromycin ethyl succinate (an ester). These two preparations are still susceptible to acid inactivation. Despite the fact that they are marketed with a buffering agent or as film-coated or entericcoated tablets, they should be administered at least 1 hour before a meal.
3. Propinyl erythromycin ester lauryl sulfate (erythromycin estolate) (the salt of an ester).
4. Stearate salt of 2u-acetyl ester of erythromycin (erythromycin ascitrate) . These last two formulations are more resistant to inactivation by gastric acid and can be administered in the fasting state or after food.
Subsequent to the development of erythromycin, a series of semisynthetic compounds with improved stability in an acidic environment and oral bioavailability, as well as markedly improved pharmacologic profiles, have been developed – these include clarithromycin, roxithromycin, and azithromycin.
In addition, some older macrolides, including spiramycin, josamycin, and rosaramycin, remain available in some regions. The availability of clarithromycin, roxithromycin, and azithromycin has substantially reduced the use of erythromycin over the last decade. Erythromycin is mainly active against Gram-positive cocci, as well as against a few Gram-negative bacteria, including Neisseria spp., Haemophilus spp., Legionella spp., as well as Chlamydia spp. and Mycoplasma spp.
You may like
Related articles And Qustion
Lastest Price from Erythromycin manufacturers

US $0.00/kg2025-04-23
- CAS:
- 114-07-8
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg

US $0.00/Kg/Drum2025-04-21
- CAS:
- 114-07-8
- Min. Order:
- 1KG
- Purity:
- 93%-102%; EP
- Supply Ability:
- 500KGS