Toxicity of Moxifloxacin
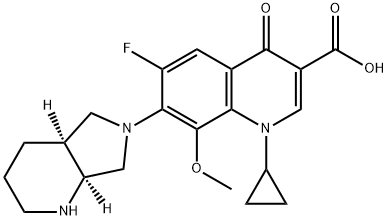
Moxifloxacin (Bay 12-8039) is a fourth-generation synthetic fluoroquinolone developed by Bayer Pharmaceuticals in the early 1990s. It has a molecular weight of 401.431 and has the chemical formula 1-cyclopropyl-7-[(1S,6S)-2,8-diazabicyclo[4.3.0]non-8-yl]-6-fluoro-8- methoxy-4-oxo-quinoline-3-carboxylic acid. Its empirical formula is C21H24FN3O4.

Moxifloxacin (as moxifloxacin hydrochloride) is available under the brand name Aveloxs as 400-mg tablets. It is also available in parenteral form for intravenous infusion. Moxifloxacin is also sold in an ophthalmic solution 0.5% (eye drops) under the name Vigamoxs. Moxifloxacin inhibits bacterial topoisomerase II (DNA gyrase) and topoisomerase IV, and is bactericidal. Moxifloxacin has a broad spectrum of activity and is approved for use in acute bacterial sinusitis, acute bacterial exacerbations of chronic bronchitis, communityacquired pneumonia, and uncomplicated skin and skin structure infections. Moxifloxacin is also used for the treatment of complicated intra-abdominal infections, as a secondline agent in the treatment of tuberculosis and for bacterial conjunctivitis (ophthalmic solution).
Mechanism of action
The quinolones are direct inhibitors of bacterial synthesis. Like the other quinolones, moxifloxacin inhibits two bacterial enzymes, topoisomerase II (DNA gyrase) and topoisomerase IV, which are important in DNA replication. The drug binds to these enzymes, blocks DNA replication, and results in bacterial cell death.
Drug interactions
Some fluoroquinolones (e.g. enoxacin) are inhibitors of the cyp enzyme system and may produce significant drug interactions, but moxifloxacin is free of this effect. Like other fluoroquinolones, moxifloxacin is sensitive to the presence of multivalent cations, thus, absorption of moxifloxacin is decreased by 40% with concomitant administration of iron supplements, and by 60% with antacids containing aluminum or magnesium ions. For this reason the manufacturer recommends administration 4 h before or 8 h after these cation-containing compounds. Pharmacokinetic studies have shown that moxifloxacin does not interact with food, ranitidine, theophylline, itraconazole, morphine, oral contraceptives, or calcium. The potential for alteration in glucose homeostasis exists.
Toxicity
Overall, moxifloxacin has a similar safety profile to other commonly used fluoroquinolones, except for its slightly greater propensity to cause cardiac toxicity in some predisposed patients. Nevertheless, increasing patient age does not appear to be associated with an increased risk of side-effects compared with other comparator agents (Andriole et al., 2005). Even patients treated for TB with prolonged courses of moxifloxacin appear to experience relatively low rates of significant side-effects .
Neurotoxicity
Adverse reactions of the central nervous system (CNS) are a wellknown complication of quinolone therapy and occur at an overall incidence of 1–2%. Of these, the most commonly reported include dizziness, headache, and somnolence. Dizziness was reported in 2.8% of patients in the phase II and III trials of moxifloxacin with a discontinuation rate of 0.5%. Headache was noted in 1.1%. Moxifloxacin lacks the specific structure–toxicity relationships noted to induce seizures by other quinolones but should still be used with caution in patients with a history of seizure disorders.
You may like
Lastest Price from Moxifloxacin manufacturers

US $0.00/kg2025-04-24
- CAS:
- 151096-09-2
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg

US $0.00-0.00/Kg/Drum2025-04-21
- CAS:
- 151096-09-2
- Min. Order:
- 1Kg/Bag
- Purity:
- 98%-102% HPLC
- Supply Ability:
- 500kg