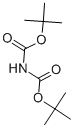
What is Di-tert-butyl iminodicarboxylate?
Feb 17,2020
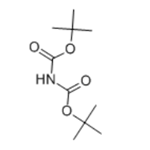
Di-tert-butyl-iminodicarboxylate is an organic compound that can be described with the formula [(CH3)3COC(O)]2NH. It is a white solid that is soluble in organic solvents. The compound is used as a reagent for the preparation of primary amines from alkyl halides. It was popularized as an alternative to the Gabriel synthesis for the same conversion. Amines can also be prepared from alcohols by dehydration using the Mitsunobu reaction. In the usual implementation the reagent is deprotonated to give the potassium salt, which is N-alkylated. The Boc protecting groups are subsequently removed under acidic conditions.
The following example is about its application on the synthesis of di-tert-butyl allylimino dicarboxylate[1].
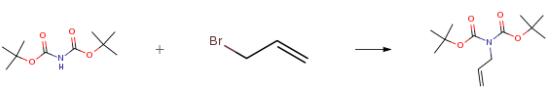
To an appropriate round bottom flask with 150 mL of 2-methyltetrahydrofuran ("2- methylthf") was added di-tert-butylimino-dicarboxylate (25.0 g, 115 mmol), allyl bromide (16.7 g, 12.0 mL, 138 mmol), and terabutylammonium bromide (0.520 g 1.61 mmol). in a second flask a sodium hydroxide solution is prepared by adding sodium hydroxide pellets (23.0 g, 576 mmol) to 100.0 mL of process water at 0°-5°C. At room temperature the solution of sodium hydroxide is added to the reaction. The reaction is heated 40°-50°C. After 1 hour HPLC (GTP 6354.01 Armor C-18 5 uM 150 x 4.6 cm, 20mM K2HPO4-PH 7), showed total consumption of di-tert-butylimino-dicarboxylate. Separated the layers and the 2-methylthf layer is washed with process water. The organic layer is displaced with isopropanol to a KF of 0.1-0. 2percent and used as a solution in isopropanol for the next step. HPLC Method on HP1100 using GTP 6354.01 indicated a main product band at 26.4 minutes with area percent of 96.0 percent. The yield was 98 percent.
The following example is about its application on the synthesis of Di(tert-butyl) 2-(2,2-dimethyl-4H-1,3-benzodioxin-6-yl)-2-oxo ethylimido dicarbonate [2].
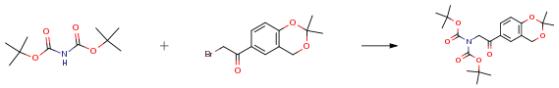
The starting material (1.0 eqt), di-t-butyl imidino dicarboxylate (1.0 eqt) and cesium carbonate (1.2 eqt) were mixed together at 20-25° C. in acetonitrile under nitrogen over a period of 6 hr. After completion of reaction, the reaction mass was filtered off and washed with acetonitrile and concentrated under vacuum below 50° C. The residue thus obtained was dissolved in toluene and washed with water followed by brine. The organic fractions were dried over anhydrous sodium sulfate and were concentrated under vacuum below 60° C. to obtain the crude product. The crude compound was slurried with diisopropyl ether, filtered and dried under vacuum at 40-45° C. to obtain the final compound. Yield: 64 percent; purity by HPLC: 99.57 percent.
The following example is about its application on the synthesis of Di-tert-butyl [(1R,4S)-4-hydroxycyclopent-2-en-1-yl]imidodicarbonate [3].
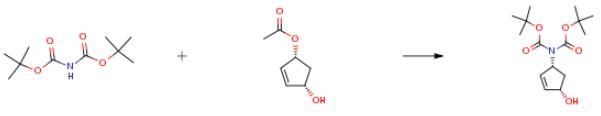
A suspension of sodium di-tert-butyl-iminodicarboxylate, previously prepared by reaction of di-tert-butyliminodicarboxylate (1.195 g, 5.50 mmol) with NaH (60percent suspension in mineral oil)(132 mg, 5.50 mmol) in dry THF (18 mL), was cannulated to a room temperature solution of (1R,4S)-4-hydroxycyclopent-2-enyl acetate(521 mg, 3.67 mmol), PPh3 (144 mg, 0.55 mmol) and Pd(PPh3)4 (636 mg, 0.55 mmol) in dry THF/DMF(1:1)(16 mL). The reaction mixture was heated at 50 °C for 1 day, then diluted with MeOH (10 mL) and dryloaded on to silica. Chromatographic purification yielded 17 (463 mg, 42 percent) as a colourless oil that solidifies upon standing to afford a white solid.
References
1.Pfizer products inc. Processes for the preparation of n-((((pyridinyloxy) -phenylamino) quinazolinyl)- allyl) acetamide derivatives and related compounds as well as intermediates of such processes and processes for the preparation of such intermediates. WO2004/89934[P], 2004, A1, Location in patent: Page 25
2.Perrigo Api Ltd.; Dammalapati VLNR, Mudduluru HK, Aduri R. Process for the preparation of vilanterol and intermediates thereof. US2015/239862[P], 2015, A1, Location in patent: Paragraph 0127; 0128
3.Llona-Minguez S, Mackay SP. Stereoselective synthesis of carbocyclic analogues of the nucleoside Q precursor (PreQ0)[J]. Beilstein Journal of Organic Chemistry, 2014, 10:1333 - 1338
See also
Lastest Price from Di-tert-butyl iminodicarboxylate manufacturers
Di-tert-butyl iminodicarboxylate

US $0.00/KG2025-04-15
- CAS:
- 51779-32-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg
Di-tert-butyl iminodicarboxylate
US $0.00-0.00/KG2025-04-04
- CAS:
- 51779-32-9
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1ton