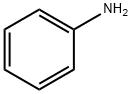
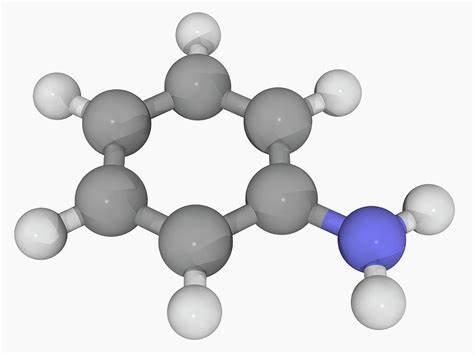
What is Aniline?
Aniline is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds.
Chemically, it is considered an electron-rich benzene derivative, and as a consequence, reacts rapidly in electrophilic aromatic substitution reactions. Likewise, it is also prone to oxidation: while freshly purified aniline is an almost colorless oil, exposure to air results in gradual darkening of the sample (to yellow or red) due to the formation of strongly colored, oxidized impurities. Aniline can be diazotized to give a diazonium salt, which can then undergo various nucleophilic substitution reactions.
Production
Industrial aniline production involves two steps. First, benzene is nitrated with a concentrated mixture of nitric acid and sulfuric acid at 50 to 60 °C to yield nitrobenzene. The nitrobenzene is then hydrogenated (typically at 200–300 °C) in the presence of metal catalysts:
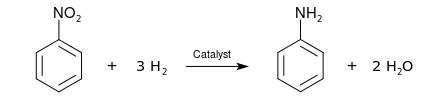
The reduction of nitrobenzene to aniline was first performed by Nikolay Zinin in 1842, using inorganic sulfide as a reductant (Zinin reaction). The reduction of nitrobenzene to aniline was also performed as part of reductions by Antoine Béchamp in 1854, using iron as the reductant (Bechamp reduction).
Aniline can alternatively be prepared from ammonia and phenol derived from the cumene process.
In commerce, three brands of aniline are distinguished: aniline oil for blue, which is pure aniline; aniline oil for red, a mixture of equimolecular quantities of aniline and ortho- and para-toluidines; and aniline oil for safranine, which contains aniline and ortho-toluidine and is obtained from the distillate (échappés) of the fuchsine fusion.
Related aniline derivatives
Many analogues of aniline are known where the phenyl group is further substituted. These include toluidines, xylidines, chloroanilines, aminobenzoic acids, nitroanilines, and many others. They often are prepared by nitration of the substituted aromatic compounds followed by reduction. For example, this approach is used to convert toluene into toluidines and chlorobenzene into 4-chloroaniline.[6] Alternatively, using Buchwald-Hartwig coupling or Ullmann reaction approaches, aryl halides can be animated with aqueous or gaseous ammonia.[14]
Reactions
The chemistry of aniline is rich because the compound has been cheaply available for many years. Below are some classes of its reactions.
Uses
Aniline is predominantly used for the preparation of methylenedianiline and related compounds by condensation with formaldehyde. The diamines are condensed with phosgene to give methylene diphenyl diisocyanate, a precursor to urethane polymers.
Other uses include rubber processing chemicals (9%), herbicides (2%), and dyes and pigments (2%).As additives to rubber, aniline derivatives such as phenylenediamines and diphenylamine, are antioxidants. Illustrative of the drugs prepared from aniline is paracetamol (acetaminophen, Tylenol). The principal use of aniline in the dye industry is as a precursor to indigo, the blue of blue jeans.
Related articles And Qustion
Lastest Price from Aniline manufacturers

US $2.70/kg2025-06-06
- CAS:
- 62-53-3
- Min. Order:
- 10kg
- Purity:
- 99%
- Supply Ability:
- 10000kg

US $10.00/KG2025-04-21
- CAS:
- 62-53-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt