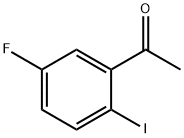
What is 5'-fluoro-2'-Iodoacetophenone?
The 5'-Fluoro-2'-iodoacetophenone, its CAS number is 914225-70-0, and it also can be called as 1-(5-Fluoro-2-iodophenyl) ethanone.
Cancer is a major cause of death in humans from disease, and there are many types of cancer. One of the leading causes of death in the world. Cancer is caused by a series of complex multiple genetic and molecular events, including gene mutations, chromosomal translocations and other factors. In order to meet the needs of treating diseases, the research and development of anticancer drugs, especially changing the molecular target or targeting multiplicity during the treatment of cancer, have been the focus of pharmaceutical companies all over the world.
Studies found that ((10R)−7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H−8,4-(metheno)pyrazolo[4,3−h][2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile is a next-generation small-molecule inhibitor of the orphan receptor tyrosine kinase c-ros oncogene 1 (ROS1), which has a kinase domain that is physiologically related to anaplastic lymphoma kinase (ALK) [1]. The drugs are mainly used in clinical cancer, including lung cancer, bone cancer, skin cancer, head and neck cancer, skin or intraocular melanoma, uterine cancer, ovarian cancer, rectal cancer, stomach cancer, esophageal cancer, lymphocytic lymphoma, non-small cell lung cancer, neuroblastoma and so on. Importantly, 1-(5-fluoro-2-iodophenyl) ethyl ketone is synthesis intermediate of ((10R)−7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H−8,4-(metheno)pyrazolo[4,3−h][2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile.

Scheme 1 Synthesis of 5'-Fluoro-2'-iodoacetophenone
So how to prepare 5'-Fluoro-2'-iodoacetophenone is key to prepare anticancer drug.
The synthesis procedure was listed as below (Scheme 1) [2].
Add 2 - amino - 5 - Fluoro benzoic acid in prepared sulfuric acid solution and keep mixing at 0~10 ℃. Then drop-wise adds the sodium nitrite solution and keep stirring till no solid. TLC showed the reaction was complete. Add urea and cool down to 0℃. Keep stirring as adding 0℃ potassium iodide (KI) solution and increase temperature to room temperature. Still keep stirring as a filter and obtain brown solid, which was dissolved with ethyl acetate, and washed with hydrochloric acid, sodium bisulfate and saturated salt water, dried. After further purification and obtain 5-fluoro-2-iodobenzoic acid. In the step 2, add 5-fluoro-2-iodobenzoic acid to DMSO and heat to 60°C, and keep stirring to get clarified liquid. Steam dry solvent and add toluene to wash. Then steam dry toluene, ethyl acetate was added inside together with diethyl malonate. The solution is mixing while adding magnesium chloride at room temperature for 30 minutes, then drop-wise adds triethylamine and keep 30 minutes after adding. Cool down solution to 5 ~10°C and add the acyl chloride at room temperature for 2 hours. After reaction, 2N HCl was added in the cool solution. After a series washing, purification process,1-(5-Fluoro-2-iodophenyl) ethyl ketone was obtained through a silica gel column.
References
[1] Thomas Lee Collier, etc., Synthesis and preliminary PET imaging of 11C and 18F isotopologues of the ROS1/ALK inhibitor lorlatinib, Nature Communications volume 8, Article number: 15761 (2017)
[2] Guo, Songpo; Qiu, Dongcheng, CN 105732355, Preparation method of 1-(5-fluoro-2-iodophenyl)ethanone with 2-amino-5-fluorobenzoic acid.
You may like
Lastest Price from 5'-fluoro-2'-Iodoacetophenone manufacturers

US $0.00-0.00/KG2025-04-04
- CAS:
- 914225-70-0
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1Ton

US $0.00/kg2025-03-10
- CAS:
- 914225-70-0
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 500 kgs