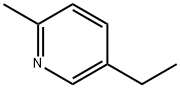
What is 5-Ethyl-2-methylpyridine?
5-Ethyl-2-methylpyridine is found in alcoholic beverages. 5-Ethyl-2-methylpyridine is present in dry red beans, cocoa, tea and whisky. 5-Ethyl-2-methylpyridine is a flavouring agent [1]. 5-Ethyl-2-methylpyridine is one of the components that contribute to the nutty, roasted aroma in the Parmigiano-Reggiano cheese.
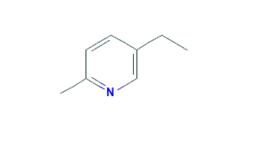
5-Ethyl-2-methylpyridine appears as a colorless to yellow liquid. Insoluble in water and more dense than water. Hence sinks in water. Contact may slightly irritate the skin, eyes, and mucous membranes. May be toxic by ingestion. Used to make other chemicals.
5-Ethyl-2-methylpyridine, associated with Maillard reaction chemistry and sugar thermal degradation processes, has been used to study the nanogram adsorption for SPME headspace and liquid sampling.
It is well known that alkylpyridines have great applications in polymer, chemical and pharmaceutical industries because of their specific reactivities and physiological activities. Among these well-known alkylpyridines, 5-ethyl-2-methylpyridine(EMP) is of great interest to be used as a raw material in the syntheses of some important fine chemical products, such as nicotinic acid, nicotinamide and 2-methyl-5-vinyl pyridine(MVP), which are good intermediates in the syntheses of drugs [2]. In addition, the applications of EMP in synthetic rubber and fertilizer synergist are popular as well.
Chemical syntheses of EMP via pyridine derivative process and amine cyclization method are unpractical due to the lack of starting materials and the difficulties in preparing catalysts. Most attention has been paid to the aldehydes(or ketones)-ammonia method[8―13], especially the condensation reaction of acetaldehyde or paraldehyde with ammonia to form EMP in the presence of accessible catalysts, such as ammonium salts, sodium carbonate and sodium fluoride, etc.. However, the above methods have some drawbacks, such as the use of homogeneous catalysts, the separation of catalyst and the selectivity of product. In view of this, Shimizu et al. and Kulkarni et al. reported the syntheses of acetaldehydes with high selectivity and high conversion using modified solid heterogeneous zeolite catalysts under high pressure conditions. Nevertheless, aldehydes are harmful to the environment and the research for an environmentally benign method for the synthesis of EMP is in demand [3].
On one hand, the unique nature of the hydrothermal condition can provide a new synthetic route in an innovative, unconventional, as well as safest way, which has been illustrated by numerous novel synthetic discoveries in the realm of hydrothermal synthesis. On the other hand, with increased availability and decreased cost, ethanol is a potentially promising platform molecule for the production of a variety of value-added chemicals owing to its increased availability and low cost [4].
TIAN Zhenzhen etc. reported a simple one-pot approach to synthesize EMP using an accessible and eco-friendly reagent, C2H5OH, instead of aldehyde, to perform alcohol-ammonia condensation reaction under hydrothermal condition. In the meanwhile, a new commercially available heterogeneous catalyst, as well as oxidant, Cu2O, was explored after investigating various kinds of different catalysts. The use of eco-friendly and cost efficient raw materials made our method valuable for the present society. Different reaction conditions were researched and the optimal ones were achieved by studying the parameters, that could affect the yield of product and by considering the energy and resource saving. The present study provided an eco-friendly way to obtaining EMP with lower volatility using fewer toxic starting materials [5].
References
[1] https://pubchem.ncbi.nlm.nih.gov/compound/5-Ethyl-2-methylpyridine
[2] Nenz A., Pieroni M., Hydrocarb. Process, 1968, 47, 139
[3] Rama Rao A. V., Kulkarni S. J., Ramachandra Rao R., Subrahmanyam M., Appl. Catal. A, 1994, 111, L101
[4] Wang Y. L., Tan D. X., Chem. Res. Chinese Universities, 2014, 30(2), 320
[5] Zhenzhen Tian, Dong Zhang, Biao Guo, Ge Tian, Xinxin Liu, Huijuan Yue & Shouhua Feng, One-step synthesis of 5-ethyl-2-methylpyridine from NH4HCO3 and C2H5OH under hydrothermal condition, Chemical Research in Chinese Universities volume 31, pages249–252(2015)