ChemicalBook > Articles Catagory List >Amino-Acids-and-Derivatives >what-is-3-amino-2-naphthoic-acid-
What is 3-amino-2-naphthoic acid?
Feb 12,2020
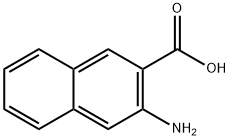
3-Amino-2-naphthoic acid, is an unnatural aromatic amino acid, that can be used for the manufacture of more complex compounds. It can be utilized for the synthesis of novel acronycine/duocarmycin hybrid natural product.
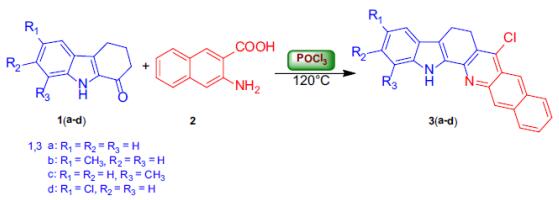
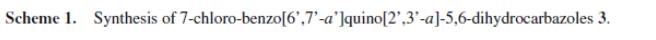
Arya et al. reported [1] its application on the synthesis of carbazole fused benzoquinolines and pyridocarbazoles via a modified Friedländer hetero-annulation reaction between 2, 3, 4, 9-tetrahydrocarbazol-1-one and 3-amino-2-naphthoic acid in the presence of POCl3, The direct pseudo multicomponent transformation of 2, 3, 4, 9-tetrahydrocarbazol-1-one, malononitrile and 9-ethyl-3-carbazolecarboxaldehyde results in the formation of a multifunctionalized carbazole through a Knoevenagel–Michael addition-cyclization reaction has also been found. The heteroarylcarbazole derivatives have been found to display a diverse array of important functions.
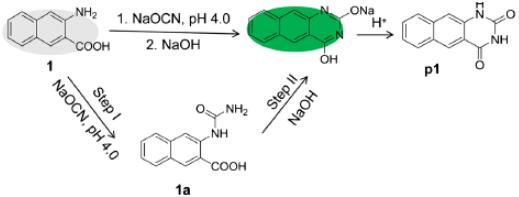
Feng et al. reported [2] its application on the synthesis of 3-amino-2-naphthoic acid-based turn-on fluorescence probe. Upon the addition of sodium cyanate, the weak-fluorescent 3-amino-2-naphthoic acid could react with CNO−, which triggered intense emission of green fluorescence. And up to 9-fold fluorescence enhancement was observed. The fluorescence enhancement ratios displayed a good linear relationship with the concentrations of CNO− in the range of 0.5–200 μM. The high selectivity and sensitivity for CNO− detection were investigated with the detection limit as low as 260 nM. The probe was further successfully applied to determine CNO− in real samples such as tap water, human urine and serum samples, which offered a promising approach in practical applications.
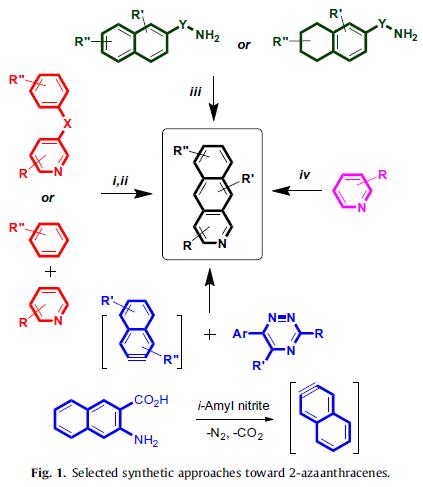
Kopchuk et al. reported [3] its application on the synthesis of 3-cyano-2-azaanthracene-based ‘‘push-pull” fluorophores via a facile one-step approach towards 3-cyano-2-azaanthracenes via the [4+2] cycloaddition reaction between 5-cyano-1,2,4-triazines and 2,3-dehydronaphthalene, generated in situ from commercially available 3-amino-2-naphthoic acid. Only 1,2,4-triazines activated by the electronwithdrawing cyano-group at the C5 position were successfully involved as dienes in this reaction, affording the corresponding cyano- and aryl-substituted 2-azaanthracenes, which were isolated in moderate yields. The 2-azaanthracene moiety features in molecules which have been used as cellular stains, pH sensors, environmentally sensitive dyes, metal cation sensors and ligands.
References
1.Arya KR et al. An efficient one-pot synthesis of carbazole fused benzoquinolines and pyridocarbazoles[J]. J. Chem. Sci. 2018, 130:41
2.Feng et al. Selective and sensitive detection of cyanate using 3-amino-2-naphthoic acid-based turn-on fluorescence probe[J]. Analytical and Bioanalytical Chemistry, 2019, 411:3613–3619
Kopchuk DS et al. 3-Cyano-2-azaanthracene-based ‘‘push-pull” fluorophores: A one-step preparation from 5-cyano-1,2,4-triazines and 2,3-dehydronaphthalene, generated in situ[J]. Tetrahedron Letters, 2016, 57:5639–5643
You may like
Application research of Hexapeptide 9
Sep 4, 2025
The study of Fmoc-L-Arg(Pbf)-OH
Aug 15, 2025
Related articles And Qustion
Lastest Price from 3-Amino-2-naphthoic acid manufacturers
3-Amino-2-naphthoic acid

US $45.00/kg2025-04-21
- CAS:
- 5959-52-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 tons
3-Amino-2-naphthoic acid

US $1.00/KG2019-12-31
- CAS:
- 5959-52-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200kg