ChemicalBook > Articles Catagory List >Inorganic-chemistry >what-happens-when-calcium-carbonate-reacts-with-acid
What Happens When Calcium Carbonate Reacts with Acid?
Mar 13,2024
What happens when you put calcium carbonate in hydrochloric acid?

The chemical reaction is as follows:
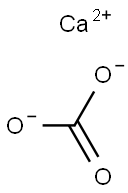
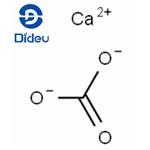
CaCO3 + 2HCl==> CaCl2 + CO2 + H2O
In this chemical reaction, calcium carbonate reacts with the acid to yield calcium chloride and carbon dioxide gas, which escapes as bubbles. The resulting calcium chloride is highly soluble in water, facilitating the dissolution of calcium carbonate by the acid.
You may like
Related articles And Qustion
See also
Lastest Price from Calcium carbonate manufacturers
Calcium carbonate
US $0.00-0.00/kg2025-12-13
- CAS:
- 471-34-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000
Calcium Carbonate

US $1200.00-1100.00/ton2025-09-10
- CAS:
- 471-34-1
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M