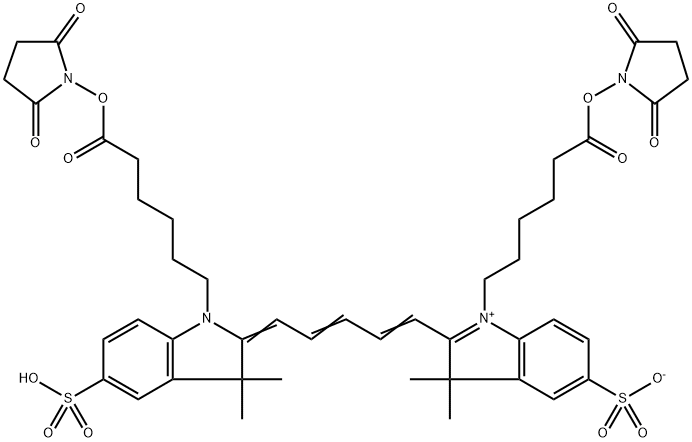
What are the effects and precautions of dicyanin dye?
Derived from the depths of coal tar, dicyanin emerges as a captivating dye with the remarkable ability to unveil light waves beyond the constraints of human vision. This unparalleled feat is made possible by the phenomenon of fluorescence, wherein the dye absorbs light energy, often extending beyond the visible spectrum, and promptly emits it as visible light, thus illuminating the previously unseen.
The mechanism behind dicyanin's extraordinary capabilities lies within its molecular structure and its interaction with light. Acting as a spectral alchemist, dicyanin absorbs a spectrum of light waves, including ultraviolet radiation, and seamlessly converts this absorbed energy into visible light. This process unfolds swiftly, casting its surroundings in an ethereal glow.
Despite its luminous properties, dicyanin harbors a clandestine truth—it bears inherent toxicity. This revelation presents a significant challenge, impeding its widespread availability and constraining its applications. Nevertheless, the allure of its spectral enchantment continues to captivate researchers and enthusiasts, compelling them to unlock its full potential while navigating the safety concerns it entails.
While dicyanin may evoke visions of ghostly apparitions and celestial radiance, its essence is firmly grounded in the principles of chemistry and physics. Belonging to the broader family of cyanine dyes, each member harbors its own unique spectral signature and offers diverse potential applications.