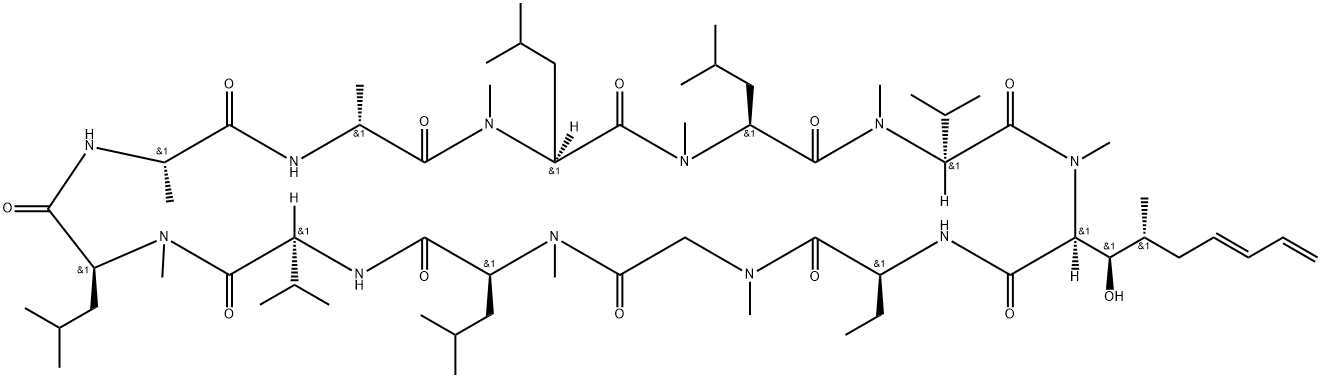
Voclosporin: Synthesis and Description
Synthesis of Voclosporin
Voclosporin is synthesised using intermediate cyclosporin A as raw material by chemical reaction. The specific synthesis steps are as follows:
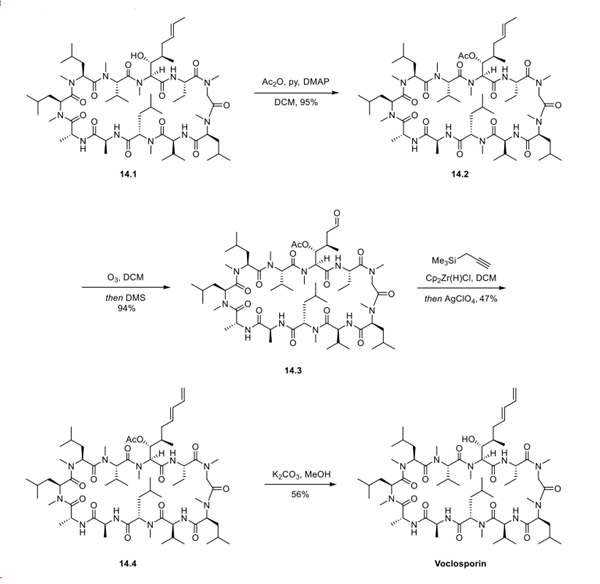
The synthesis of voclosporin started from the advanced intermediate cyclosporin A 14.1, which is a natural product that can be accessed via fermentation. Exposure of cyclosporin A 14.1 to acetic anhydride, pyridine, and DMAP in DCM provided acetyl cyclosporin A 14.2 in excellent yield. Intermediate 14.2 then underwent ozonolysis to provide the oxidation adduct 14.3. Propargyltrimethylsilane and bis(cyclopentadienyl)zirconium chloride hydride were utilized in the presence of silver perchlorate to homologate aldehyde 14.3, providing trans-alkene 14.4. Lastly, base-mediated acetyl removal generated voclosporin following semipreparative high-pressure liquid chromatography (HPLC) purification.
Description of Voclosporin
Voclosporin is a calcineurin inhibitor immunosuppressant that received its first approval by the USFDA for the treatment of active lupus nephritis, making it the first oral therapy approved in the US for this indication. Lupus nephritis can result in permanent kidney damage and is a complication of systemic lupus erythematosus. Treatment approaches for lupus nephritis generally aim to optimize kidney function and prevent kidney failure. Voclosporin was developed by Aurinia Pharmaceuticals and is dosed in adults in combination with background immunosuppressive therapy.
You may like
See also
Lastest Price from Voclosporin manufacturers
US $0.00-0.00/kg2025-08-22
- CAS:
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1