Virginiamycin: Animal Growth Promotion and the Human Health Risk Quandary
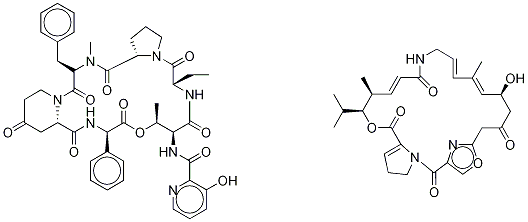
Virginiamycin is defined as a streptogramin antibiotic used in animals to promote growth, which has been associated with the emergence of antibiotic-resistant Enterococcus faecium. Virginiamycin inhibits protein synthesis by binding to the 23S ribosomal subunit and blocking the translation process, with no effect on transcription. It is not commonly used to treat bacterial infections in domestic animals despite possessing a broad-spectrum antimicrobial activity. Most often it is used as a feed-additive formulation for growth promotion in animals such as swine, turkey, and broiler chickens.

Scaling up a virginiamycin production by a high-yield Streptomyces
Virginiamycin, an antibiotic produced by Streptomyces virginiae, belongs to the streptogramin group and represents a natural mix of two synergistic factors, M (hybrid polyketide-peptide) and S (peptide). The main antimicrobial components of the antibiotic are the M1 and S1 factors. One of the unique features of the virginiamycin biosynthesis is a simultaneous production of both M1 and S1 at a ratio providing their maximum synergistic activity in the suppression of the protein biosynthesis in susceptible microorganisms. The maximum synergism is observed when M1 and S1 are present in the optimum ratio of 70–75:25–30; in this case, the total antimicrobial activity of virginiamycin increases 3.5–4 times. There is also another point of view based on the study of antimicrobial characteristics of quinupristin/dalfopristin, another antibiotic of the virginiamycin group, at different ratios of its components (Jamjian et al. 1997). This study showed no significant difference between various quinupristin/dalfopristin ratios. Nevertheless, there were no similar studies concerning virginiamycin, i.e., the idea of the best synergistic ratio of its components still has not been disproved. An increased productivity of industrial antibiotic-producing strains often results in a self-inhibiting effect of their metabolites able to suppress not only their own biosynthesis, but also the growth and development of a microorganism.[1]
Thus, we successfully scaled-up the technology of a virginiamycin production by high-yield S. virginiae VKM Ac-2738D from 50-ml shake flasks to a 100-L stirred fermenter and reproduced on a pilot-scale results obtained on a lab-scale. Different types of synthetic resins were first evaluated for their ability to reduce the self-inhibition effect and improve the virginiamycin titer in a lab-scale, and an optimal variant was successfully tested in a pilot-scale. The developed technology provides a final virginiamycin titer at the level of 5.6 g/L and has several additional advantages. First, VKM Ac-2738D produces virginiamycin M1 and S1 at the optimum ratio providing the maximum antimicrobial activity of a final product; the performed scale-up kept this important parameter unchanged. Second, VKM Ac-2738D is able to utilize sucrose, which represents less expensive carbon source than traditionally used glucose or d-maltose. Third, the used adsorbing resin, Diaion® HP21, is able to selectively bind up to 98.5% of this medicine from culture broth that simplifies the further recovery procedure. The data obtained during this study provide some new information and can be used in other virginiamycin-related studies.
Human Health Risks from Virginiamycin Use in Food Animals in China
Farm animals in many parts of the world test positive for QD-resistant E. faecium (SREF), raising concern about the theoretical possibility that use of Virginiamycin on farms might compromise QD effectiveness in treating human VREF infections. Whether it does so in reality has proved difficult to test, in part because of uncertainty about the extent to which streptogramin resistance genes or determinants transfer from bacteria in animals to VREF in humans under realistic conditions. However, if widespread resistance eventually develops to linezolid and other emerging treatment options for VREF infections, such as daptomycin-based therapies, then it is possible to speculate that QD might at some future date be approved for use in treating VREF infections in human patients in China. If this were to happen, and if continuing Virginiamycin use were to increase QD resistance in VREF infections not treatable by other means such as linezolid and daptomycin, then discontinuing VM use now might create a future human health benefit from reduced QD resistance in VREF infections. The following sections apply QRA methods to estimate how large this potential human health benefit might be. The analysis presented here has undertaken such a quantitative examination for the animal antibiotic VM in China.[2]
To deal with realistic data gaps and uncertainties, we expressed potentially avoidable human health risk as a product of factors and estimated various plausible values, ranges of values, or upper bounds for each of them. A key contributor to this cost outside of China is now recognized to be the withdrawal of antibiotics, including Virginiamycin, from use other than disease treatment. Even if continuing use of VM reduces NE costs that would otherwise occur in China by only 10%, or about US$37M/year; and even if each statistical life mortality prevented or postponed were valued at $10M and one excess mortality per year could be prevented every year instead of ever few decades to thousands of years, the estimated cost of discontinuing Virginiamycin would still substantially outweigh the estimated monetized benefits based on these calculations. We conclude that appeals to restrict animal antibiotic or antimicrobial use based on concerns about preserving antibiotic efficacy for human medicine do not provide sufficient information about the probable consequences of restrictions to inform rational and effective risk management policy making. Quantitative assessment is essential. For the case of Virginiamycin considered here, the potential value of hypothesized human health benefits to be gained by restricting VM use in animals appears to be small compared to the potential costs from increased animal diseases.
References
[1]Dzhavakhiya V, Savushkin V, Ovchinnikov A, Glagolev V, Savelyeva V, Popova E, Novak N, Glagoleva E. Scaling up a virginiamycin production by a high-yield Streptomyces virginiae VKM Ac-2738D strain using adsorbing resin addition and fed-batch fermentation under controlled conditions. 3 Biotech. 2016 Dec;6(2):240. doi: 10.1007/s13205-016-0566-8. Epub 2016 Nov 12. PMID: 28330311; PMCID: PMC5234532.
[2]Cox, Louis Anthony Jr et al. “Quantifying Human Health Risks from Virginiamycin Use in Food Animals in China.” Risk analysis : an official publication of the Society for Risk Analysis vol. 40,6 (2020): 1244-1257. doi:10.1111/risa.13466
Related articles And Qustion
See also
Lastest Price from Virginiamycin manufacturers

US $10.00/KG2025-04-21
- CAS:
- 11006-76-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $150.00/kg2025-04-21
- CAS:
- 11006-76-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 500kg