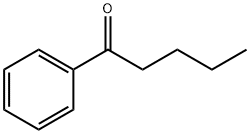
Valerophenone: Reactivity and Safety
General Description
Valerophenone is an aromatic ketone with a pentanoyl group in its benzene ring structure and is a volatile oil component and plant metabolite. However, it is not used in perfumes and is commonly used as a tool for the study of various photochemical processes. It is also commonly used as an intermediate in organic synthesis for the preparation of other aromatic compounds. It is also an inhibitor of carbonyl reductase.

Figure 1. Valerophenone
Reactivity
Norrish type reactions
Studies have demonstrated a dramatic change in the photoreactivity of Valerophenone between cyclopropyl and carbonyl at the R position. The nature of the medium significantly alters the kinetics and product distribution. High dielectric and polar solvents affect the relative energies of the 3 nπ* and 3 ππ* excited states. Norrish-type reaction studies of valerophenone in aqueous solution show that in the complex of valerophenone with water, the formation of intermolecular hydrogen bonds leads to a blue shift of the n,π* excited state, whereas Coulombic interactions between valerophenone and the surrounding aqueous humor are the main cause of the red shift of the π,π* excited state. As a result, the 3ππ* state becomes the lowest triplet state, which explains the long lifetime of the triplet state of aqueous valerophenone.
The 1nπ*, 3ππ*, and 3nπ* states were found to intersect in the same structural region, which appears to be the main reason why the intersystem crossing from 1nπ* to 3nπ* is very efficient for aqueous valerophenone and why most aromatic ketones have unique photophysical features such as a short singlet lifetime, high phosphorescence, and weak fluorescence. The Coulomb interaction between valerophenone and the bulk surrounding water has a significant influence on the α-C?C cleavage and the 1,5-H shift reaction.
Photoreactivity of Valerophenone in frozen solid solvents
Photoreactivity studies on Valerophenone in frozen solid solvents (benzene, cyclohexane, tert-butanol, hexadecane and water) have been reported. A portion of the ketone molecules are almost unreactive in the solid state due to the physical constraints of the solid solvent cavity. Free rotation along the C-C bond in the cavity becomes difficult and larger conformational changes may be completely limited. It was shown that a fraction of the molecules with conformations favourable for hydrogen abstraction can react with the same photochemical efficiency regardless of the solvent used. Furthermore, in a study of the elimination/cyclisation ratios as a function of temperature for the Norrish type II reaction, it was found that the change in elimination/cyclisation ratios specific to each solvent decreased with decreasing temperature.
Safety
Valerophenone is toxic to humans and is a strong eye and skin irritant. Inhalation or exposure to Valerophenone may cause respiratory irritation. Although it has many chemical applications. However, it is not approved for use as a chemical in New Zealand.
References:
[1] LINA DING. Solvent Effects on Photoreactivity of Valerophenone: A Combined QM and MM Study[J]. Journal of Organic Chemistry, 2009, 74 23: 8909-9244. DOI:10.1021/jo902080z.[2] LIN SHEN W H F. The Reactivity of the 1,4-Biradical Formed by Norrish Type Reactions of Aqueous Valerophenone: A QM/MM-Based FEP Study[J]. Journal of Organic Chemistry, 2011, 76 3: 749-1004. DOI:10.1021/jo101785z.
See also
Lastest Price from Valerophenone manufacturers

US $0.00/kg2026-03-02
- CAS:
- 1009-14-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 99999

US $0.00-0.00/KG2025-12-23
- CAS:
- 1009-14-9
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS