4-Bromobiphenyl: Electronic Properties and Toxicity
The 4-bromobiphenyl is the key intermediate of many fine chemical products such as laser dyes, liquid crystal material, agricultural chemicals, medicine, can make alkyl biphenyl, cyclohexyl biphenyl by the 4-bromobiphenyl, introduces cyano group and just makes biphenyl cyanogen class liquid crystalline cpd commonly used.

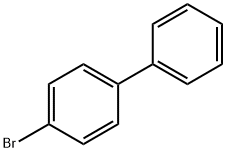
Figure 1. 4-Bromobiphenyl
4-bromobiphenyl does not also have producer to grasp the technology of this product large-scale production at home, has only part colleges and universities to make this product in the laboratory, brominated amount, the Oranoleptic indicator is last with external product have certain gap.Retrieve external pertinent literature, top grade product content is 98.0%, 91.5-92 ℃ of fusing points.
The preparation method of 4-bromobiphenyl routine carries out the Gomberg reaction with para-bromoaniline and benzene, and para-bromoaniline then prepares by Acetanilide.The shortcoming of this method is that synthesis path is long, and yield low (about 35%), product are put variable ash for a long time.
Japan Chisso Corporation (JP) 6-32, Nakanoshima 3-chome, Kitaku, Osaka, Japan once used direct bromo legal system to be equipped with the 4-bromobiphenyl, had been to react in the polarity of solvent environment with acetic acid, and yield only is 34%.Direct bromination in the non-polar solvent tetracol phenixin such as the Stone brightness of department of chemistry, tsinghua university, prepares the 4-bromobiphenyl, and yield only is 32%.
Preparation method of 4-bromobiphenyl
The objective of the invention is to propose the preparation method of the 4-bromobiphenyl that a kind of production cost is low, yield is high.
In order to realize the foregoing invention purpose, the present invention comprises following steps:
(1) under normal temperature and the agitation condition, biphenyl is joined 1-5 doubly in the ethylene dichloride solvent of biphenyl quality, subsequently, add the catalyzer of the 1%-25% of biphenyl quality in this solvent, catalyzer is aluminum chloride, iron trichloride, zinc chloride, iron powder or aluminium powder, after 5-30 minute, be cooled to 0 ℃, in 0.2-3 hour, add 0.1-1 doubly to the liquid bromine of biphenyl quality in this solvent again, this moment, mixture was scarlet;
(2) stir and 0-10 ℃ of condition under, feed chlorine in the mixture in step (1), the mixture color shoals gradually, and sampling is with the gas chromatograph monitoring, when the biphenyl reactivity reaches 60%-75%, stop to feed chlorine, then, be warming up to 50-75 ℃, under this temperature condition, continue reaction and stop after 2-12 hour stirring, filtering reacting liquid gets filtrate;
(3) under normal temperature and the agitation condition, filtrate is the sodium sulfite solution washed twice of 3-10% to the concentration of biphenyl quality doubly through 2-10, and with the mixed solution natural sedimentation layering of twice washing gained, the upper strata is inorganic liquid, and lower floor is an organic liquor;
(4) with the organic liquor in the step (3) in ℃ distillation down of normal pressure, 84-135, the residuum after the distillation is a 4-bromobiphenyl crude product;
(5) rectifying 4-bromobiphenyl crude product is collected the rectifying product of 115 ℃ of-135 ℃/absolute pressure 100Pa-310Pa;
(6) the rectifying product through 1-5 doubly to the concentration of rectifying product quality be 80-95% ethyl alcohol recrystallization once, the crystallization feed liquid is centrifugal through whizzer, obtains 4-bromobiphenyl wet feed;
(7) 4-bromobiphenyl wet feed toasted 2-6 hour under 400-700Pa vacuum tightness and 40-60 ℃ of condition, 4-bromobiphenyl finished product.
You may like
See also
Lastest Price from 4-Bromobiphenyl manufacturers

US $0.00-0.00/KG2025-06-06
- CAS:
- 92-66-0
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 10000KGS
US $0.00/KG2025-04-21
- CAS:
- 92-66-0
- Min. Order:
- 10g/Bag
- Purity:
- 99.90%
- Supply Ability:
- 20TONS