Uses of Temafloxacin
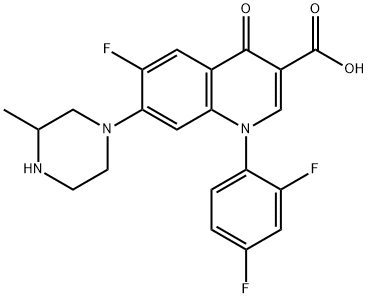
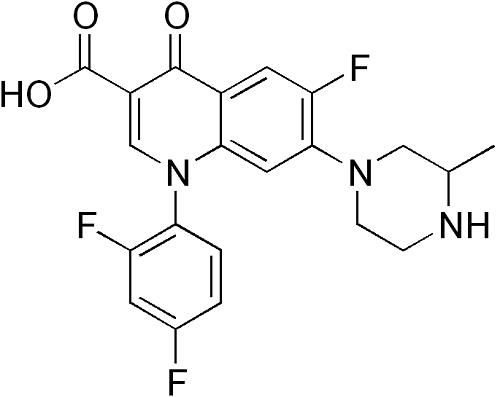
Temafloxacin (6-fluoro-7-piperazino-4-quinolone)was discontinued in June 1992, only five months after gaining marketing approval. This was due to a high rate of reported adverse reactions, including several deaths. These reactions, termed by Blum et al. as the"temafloxacin syndrome"are worthy of some discussion since there are some features that may be potentially associated, albeit at a markedly lower rate, with some other fluoroquinolones. In addition, the toxicity associated with temafloxacin highlights some of the difficulties in drug marketing since uncommon, even serious, sideeffects may escape detection despite the usual rigorous pre-market testing.
Uses
Temafloxacin was a newer generation fluoroquinolone with a broad range of activity against Gram-positive, as well as Gram-negative pathogens, and some anaerobes, with good activity against S. pneumoniae, S. aureus, Legionella, and Mycoplasma spp. Clinical studies demonstrated rates of efficacy in the treatment of lower respiratory tract infections of 90–100%, skin and soft tissue infections of W90%, urinary tract infections of W95%, prostatitis of Z84%, and excellent activity against gonococcal and non-gonococcal (other than syphilis) sexually transmitted diseases. Initially reported toxicities and drug interactions were similar to those generally associated with other fluoroquinolones, such as ciprofloxacin. It was approved by the Food and Drug Administration (FDA) for use in the treatment of lower respiratory tract infections, urinary tract infections, and skin and soft tissue infections.
Adverse reactions
Temafloxacin was withdrawn after new and serious adverse reactions were reported at a frequency of about one per 3500 patients treated. By the time of withdrawal, an estimated 189,000 prescriptions had been issued. In general, clinical trial programs can only be expected to detect adverse reactions at a frequency of about one per 1000 patients or higher, although studies using high doses may be useful in identifying dose-dependent adverse reactions. Due to the fact that only small doses of temafloxacin were necessary to induce serious reactions, early identification of the potential drug toxicity was unlikely.