Uses of Sodium cinnamate
Sodium cinnamate is a central intermediate in the biosynthesis of myriad natural products include lignols (precursors to lignin and lignocellulose), flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and phenylpropanoids. Its biosynthesis involves the action of the enzyme phenylalanine ammonia-lyase (PAL) on phenylalanine.
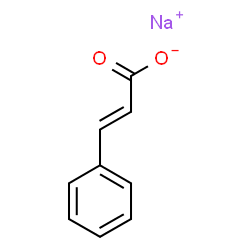
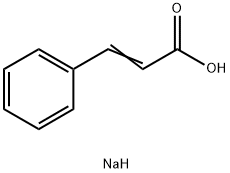
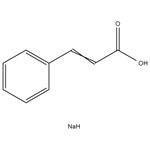
In chemistry, sodium cinnamate is an important pharmaceutical intermediate, also known as sodium 3-phenyl-2-acrylate. At room temperature it is a white to pale yellow solid that is used as an important intermediate in the manufacture of cinnamyl chloride, anticoagulant Ozagray, benzyl cinnamate, benzyl chloride and cinnamamide. Since sodium cinnamate is soluble in water, it is also a good food preservative. At the same time, it is also suitable for improving the cooling system and buffering pad of automobiles, which is beneficial for its long-distance use. At present, the common way of preparing sodium sinnamate is by the cinnamic acid and an aqueous solution of sodium carbonate reaction, and then by concentration and drying.
Cinnamic acid is used in flavorings, synthetic indigo, and certain pharmaceuticals. A major use is as a precursor to produce methyl cinnamate, ethyl cinnamate, and benzyl cinnamate for the perfume industry.[3] Cinnamic acid is a precursor to the sweetener aspartame via enzyme-catalysed amination to give phenylalanine.[4] Cinnamic acid can dimerize in non-polar solvents resulting in different linear free energy relationships.
For its pharmacological effects, recent studies have shown the pharmacological properties of cinnamic acid and its derivatives, including hepatoprotective, anti-oxidant, and anti-diabetic activities. In preclinical studies cinnamic acid demonstrated to be a promising candidate for the treatment ob obesity and diabetes. The mechanism of action of cinnamic acid in obesity is explained by its ability to inhibit lipases and ACE (angiotensin-converting enzyme).
Reference
S. Ishimatsu et al., Distribution of various nickel compounds in rat organs after oral administration. Biol. Trace Elem. Res. 49(1), 43–52 (1995).
L. T. Haber et al., Hazard identification and dose response of inhaled nickel-soluble salts. Regul. Toxicol. Pharmacol. 31, 210–230 (2000).
41. O. Norgaard, Investigations with radioactive nickel, cobalt and sodium on the resorption through the skin in rabbits, guinea-pigs and man. Acta Derm. Venereol. 37, 440–445 (1957).
G. K. Lloyd, Dermal absorption and conjugation of nickel in relation to the induction of allergic contact dermatitis: preliminary results. In S. S. Brown and F. W. Sunderman, eds., Nickel Toxicology, Academic Press, London, 1980, pp. 145–148.
You may like
See also
Lastest Price from Sodium cinnamate manufacturers

US $10.00/KG2025-04-21
- CAS:
- 538-42-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5tons
US $35.00-1.10/kg2024-04-20
- CAS:
- 538-42-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available