Uses of Dichloromethylsilane
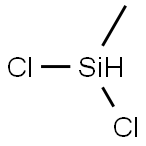
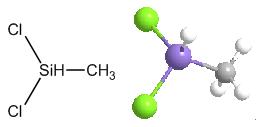
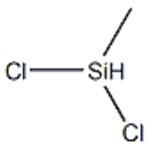
Dichloromethylsilane (methylsilicon dichloride; dichlorohydridomethylsilicon; LS 50; methyldichlorosilane; methylhydrodichlorosilane; monomethyldichlorosilane) is an organic silicon substance with a molecular formula of CH3SiHCl2 or CH4Cl2Si [1]. The molecular structure of dimethylchlorosilane contains an active Si-H bond and a Si-Cl bond, a reaction such as hydrosilylation, hydrolysis, addition, thus can be carried out to prepare a series of products such as organosilicon monomers and blocking agents, which are important raw materials for the production of ultra-pure polysilicon and in the manufacture of semiconductors and photovoltaics[2,3].
Dimethylchlorosilane and γ-chloropropene can be catalyzed by a hydrosilylation reaction to produce γ-chloropropyldimethylchlorosilane or γ-methacryloylpropyldimethylchlorosilane, which is used as blocking agents. After polymerization of blocking agents containing a reactive carbon functional group and dimethyldichlorosilane, the resulting silicone polymer end group carrying a reactive group can react with other polymers, thereby imparting new properties to improve the polymer material, physical and chemical properties [4].
Dimethylchlorosilane can be hydrolyzed to form tetramethyldisiloxane (HMM), which is an important silicone monomer. The molecular structure of HMM containing reactive oxygens at both ends, can form a difunctional siloxane monomer by a hydrosilylation reaction. The difunctional siloxane can be polymerized with a siloxane intermediate such as octamethylcyclotetrasiloxane D4 to give a functional group-containing silicone polymer and a silicone copolymer[5].
However, dichloromethylsilane is not suitable for use by the general public. The applications described take place in industrial settings under highly controlled conditions. Although the end uses of products made from dichloro(methyl)silane will vary, it can be assumed that due to its highly reactive nature, no residual unreacted material will be present in any of the final products[6].
References
[1] https://pubchem.ncbi.nlm.nih.gov/compound/methyldichlorosilane#section=Names-and-Identifiers&fullscreen=true
[2] https://www.chemicalbook.com/ChemicalProductProperty_CN_CB3854233.htm
[3] Weismann J, Gessner VH. Si-H activation by means of metal ligand cooperation in a methandiide derived carbene complex. Chem Commun (Camb). 2015, 51(80):14909-12.
[4] Zeng X, Wang C, Ge J. Study on catalytic synthesis of γ-chloropropyldimethylchlorosilane. Silicone Material. 2009, 23(2): 95-97.
[5] Colas A, Corning D. Silicones: Preparation, Properties and Performance. 2019.
[6] https://www.dow.com/content/dam/dcc/documents/en-us/compliance/01/01-43/01-4372-01-gps-safety-report-dichloro-methyl-silane.pdf?iframe=true
See also
Lastest Price from Dichloromethylsilane manufacturers
US $1.00-50.00/KG2024-03-25
- CAS:
- 75-54-7
- Min. Order:
- 1KG
- Purity:
- 99% HPLC
- Supply Ability:
- 2000 ton/year