Uses of Danthron
Danthron, a natural product, was originally extracted from
the roots and rhizome of Polygonaceae plant, also
called Da Huang in traditional Chinese herbal medicine.
Now it is synthesized in many countries, such as Germany,
India, Japan, Poland, the United Kingdom, and the United
States. Danthron is reasonably anticipated to be a human
carcinogen.
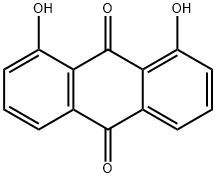
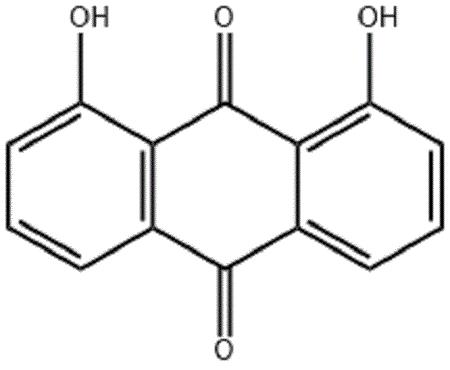
Danthron is an anthraquinone that exists at room temperature
as a red or orange crystalline powder.It is practically
insoluble in water, but soluble in a variety of solvents
(acetone, chloroform, diethyl ether, ethanol) and alkaline
hydroxide solutions. The stability of danthron is generally
good. It is stable under room temperatures and normal pressures.
Uses
Danthron has been widely administrated as a laxative since the 1900s. In the United States, danthron has been forbidden to continual use as laxative because it is considered to be a carcinogen. Food and Drug Administration (FDA) withdrew danthron from the market for this purpose in 1987. However, in the United Kingdom, it is only considered as a possible carcinogen. Therefore, danthron is only restricted to patients who have already been diagnosed as terminal cancer. Currently, danthron is being used to a lesser extent as an intermediate in the manufacture of alizarine and indanthrene dyes.
Environmental Fate
Danthron is discovered in several species of plants and insects. It has been isolated from dried leaves and stems of Xyris semifuscata harvested in Madagascar, and roots of Da Huang, a Chinese traditional herbal medicine. Danthron also appears to be biosynthesized by some insects. The presence of danthron in insects may be a way of protection from predators. Danthron can be manually synthesized by many countries. In the United States, danthron was available from 12 suppliers.
If released to the atmosphere, danthron will exist in both
the vapor phase and the particulate phase. Vapor phase danthron
has an estimated half-life of 11 days. Particulate phase
danthron can be physically removed from air by wet and dry deposition. It is expected to biodegrade with 68% degradation
within 3 months.
If released to water, danthron is expected to adsorb to the surface of solid particle and sediment. Biodegradation is also a major pathway processed in water. It was reported that 82% of the added danthron was degraded by fresh water within 3 days. If added to seawater, 91% of danthron was reported as degraded. Danthron may bioconcentrate in aquatic organisms, such as fish and shrimps.
Mechanism of Toxicity
Danthron can cause DNA damage particularly at guanines in the 5'-GG-3', 5'-GGGG-3', 5'-GGGGG-3' sequences in the presence of Cu(II), cytochrome P450 reductase and the nicotinamide adenine dinucleotide phosphate (NADPH)-generating system. H2O2 and Cu(I) may also be involved because this DNA damage can be inhibited by catalase and bathocuproine. The further mechanism is danthron is reduced by P450 reductase and generate reactive oxygen species through the redox cycle, leading to extensive Cu(II)-mediated DNA damage. The DNA damage also comes from similar topoisomerase II inhibitor behavior of danthron.
You may like
Lastest Price from 1,8-Dihydroxyanthraquinone manufacturers

US $990.00-800.00/kg2025-04-21
- CAS:
- 117-10-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000

US $45.00/kg2025-04-21
- CAS:
- 117-10-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 tons