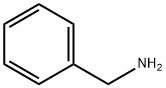
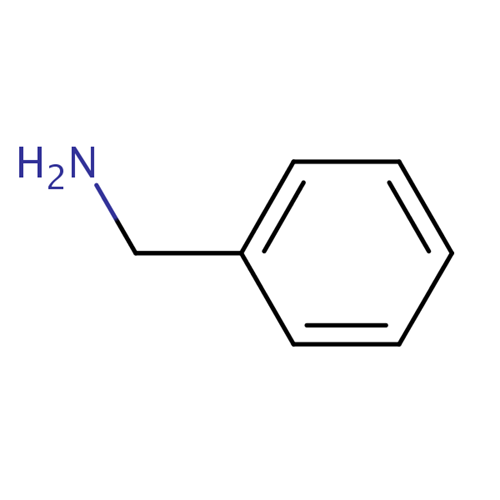
Uses of Benzylamine

Biochemistry
Benzylamine occurs biologically from the action of the N-substituted formamide deformylase enzyme, which is produced by Arthrobacter pascens bacteria.This hydrolase catalyses the conversion of N-benzylformamide into benzylamine with formate as a by-product.Benzylamine is degraded biologically by the action of the monoamine oxidase B enzyme,resulting in benzaldehyde.
Uses
Benzylamine is used as a masked source of ammonia, since after N-alkylation, the benzyl group can be removed by hydrogenolysis:
C6H5CH2NH2 + 2 RBr → C6H5CH2NR2 + 2 HBr
C6H5CH2NR2 + H2 → C6H5CH3 + R2NH
Typically a base is employed in the first step to absorb the HBr (or related acid for other kinds of alkylating agents).
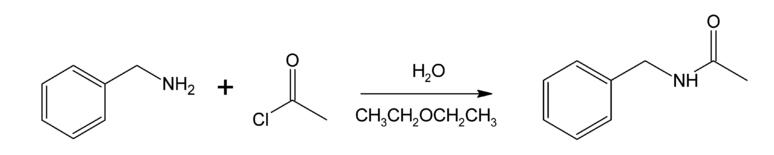
Benzylamine reacts with acetyl chloride to form N-benzylacetamide, an exemplar of the Schotten–Baumann reactionfirst described in the 1880s.The reaction takes place in a two-phase solvent system (here water and diethyl ether) so that the hydrogen chloride by-product is sequestered in the aqueous phase (and sometimes neutralised with a dissolved base) and thus prevented from protonating the amine and impeding the progress of the reaction. These conditions are often called Schotten-Baumann reaction conditions and are applicable more generally.This particular example is useful as a model for the mechanism of interfacial polymerisation of a diamine with a diacid chloride.
Safety and environment
Benzylamine exhibits modest oral toxicity in rats with LD50 of 1130 mg/kg. It is readily biodegraded.
You may like
Related articles And Qustion
See also
Lastest Price from Benzylamine manufacturers

US $10.00/KG2025-04-21
- CAS:
- 100-46-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $15.00/KG2024-10-11
- CAS:
- 100-46-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 ton