Uses of Benzidinen
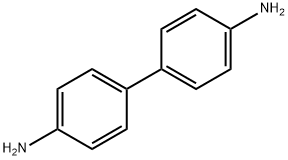
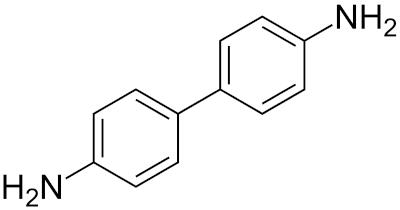
Benzidine is a biphenyl amine that exists at room temperature as a crystalline grayish-yellow, white, or reddish-gray power. It is slightly soluble in cold water, more soluble in hot water, and readily soluble in less-polar solvents such as diethyl ether and ethanol. It darkens on exposure to air and light. Benzidine is a diamine, manufactured as synthetic aromatic hydrocarbon with two benzene rings covalently bonded to one another (1,1), substituted by amino group at 4,40. Benzidine is prepared from nitrobenzene in a two-step process: nitrobenzene is converted to 1,2-diphenylhydrazine, usually using iron powder as the reducing agent, and then hydrazine is treated with mineral acids to induce a rearrangement reaction to 4,40-benzidine. In the environment, benzidine is found in either its ‘free’ state (as an organic base) or as a salt (benzidine dihydrochloride or benzidine sulfate).
Uses
Benzidine is used as an intermediate in the production of azo dyes, sulfur dyes, fast color salts, naphthol, and other dye compounds. However, it has not been marketed or sold in the United States since the mid-1970s, and US dye companies no longer manufacture benzidine-based dyes. However, a small amount of benzidine may still be manufactured or imported for scientific research in the United States, but in some countries it is still being manufactured. To date, more than 250 benzidine-based dyes have been reported. These dyes are primarily used for dyeing textiles, paper, and leather products.
Environmental Fate
Industries release benzidine into the environment in the form of liquid waste and sludges. Benzidine may also be released into the environment due to spillage during transport. In air, benzidine is found bound to suspended particles or as a vapor, which may be brought back to the earth’s surface by rain or gravity.
Mechanism of Toxicity
Benzidine is metabolized to highly toxic, reactive metabolites, such as N-hydroxyarylamides and N-hydroxyarylamines, which act as procarcinogens and are more mutagenic than parent compounds. The metabolites act as DNA adducts and bind to cell receptors. Some of the benzidine derivatives are strong mutagens. The metabolites on conjugation with sulfuric, acetic, and glucuronic acids form ultimate carcinogens. Benzidine is metabolized by cytochrome P450 enzymes (via N-oxidation) to form electrophilic compounds that can bind covalently to DNA.
Benzidine caused mutations in bacteria and plants, but gave conflicting results in cultured rodent cells. It also caused many other types of genetic damage in various test systems, including yeast, cultured human and other mammalian cells, and rodents exposed in vivo. The damage included mitotic gene conversion (in yeast), micronucleus formation, DNA strand breaks, unscheduled DNA synthesis, cell transformation, chromosomal aberrations, sister chromatid exchange, and aneuploidy. Workers exposed to benzidine and or benzidine-based dyes had higher levels of chromosomal aberrations in their white bloods cells than did unexposed workers.
You may like
Lastest Price from Benzidine manufacturers
US $0.00/kg2025-04-15
- CAS:
- 92-87-5
- Min. Order:
- 200kg
- Purity:
- 99%
- Supply Ability:
- 20 tons

US $15.00-10.00/KG2021-07-13
- CAS:
- 92-87-5
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton