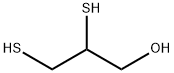
Uses of 2,3-Dimercapto-1-propanol
British Anti-Lewisite (BAL) is a synthetic therapeutic substance developed during World War II for using as an antidote against the vesicant arsenic war gases (Lewisite). The first experiments were based on the fact that arsenic products react with sulfhydryl radicals. Among all the compounds originally tested,2,3-Dimercapto-1-propanol was the most effective and the least toxic. In 1951, 2,3-Dimercapto-1-propanol was used by a renowned neurologist, Derek Denny-Brown, to treat patients suffering with Wilson’s disease (hepatolenticular degeneration), which results from excessive copper accumulation, especially in the brain and liver. The intrinsic toxicity of BAL resulted in the development of its water-soluble and less toxic derivatives dimercaptosuccinic acid and dimercaptopropanesulfonic acid.
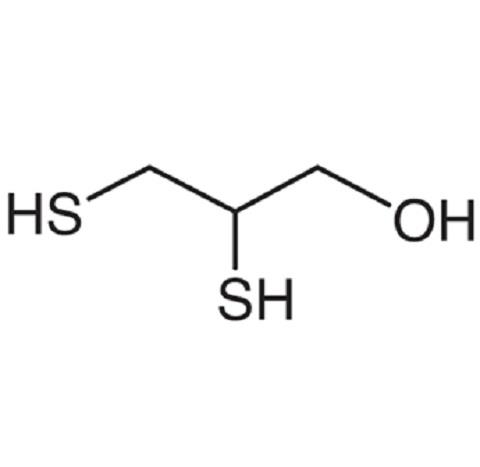
Properties
Chemically, BAL is called 2,3-dimercaptopropanol. It is a viscous oily liquid with the offensive odor of mercaptans. It is moderately soluble in water but highly soluble in vegetable oils. BAL is a dithiol compound highly reactive with arsenic and other heavy metal compounds. It has a higher affinity for these compounds than body lipids. Because BAL is unstable and susceptible to oxidation, there are storage difficulties as a readyto- use preparation. BAL is administered by deep intramuscular injection (i.m.). BAL-treated individuals suffer side effects (>50%), most of which are not serious and subside with discontinuation of treatment. BAL contains two sulfhydryl groups that react to form a stable, generally nontoxic chelate with heavy metals, particularly arsenic, mercury, lead, and tin. The combined compound prevents the metal from reacting with sulfhydryl groups in physiological proteins and thereby renders them inert until they are excreted.
Uses
2,3-Dimercapto-1-propanol is a chelating agent used as an antidote for the treatment of metal poisoning, especially arsenic (organic and inorganic), gold salts, and inorganic mercury. BAL is more effective when given soon after toxic exposure because it is more effective in preventing inhibition of sulfhydryl enzymes than in reactivating them. BAL is also used in the treatment of Wilson’s disease. The drug cannot be used in poisonings due to iron, cadmium, tellurium, selenium, vanadium, and uranium. It is contraindicated in poisonings due to elemental mercury vapor, because it can further increase the metal in the brain.
Mechanism
2,3-Dimercapto-1-propanol is believed to compete with tissue sulfhydryl groups and interferes with cellular respiration. It also competes with metallic cofactors of metabolic enzyme systems and increases capillary permeability. Metabolic degradation and excretion are essentially complete within 4 h. BAL not excreted as dimercaprol– metal complex is quickly metabolized by the liver and excreted as an inactive product in the urine. Because it is a lipophilic drug, it penetrates rapidly the intracellular spaces. The highest concentrations are found in the liver, kidneys, brain, and small intestine. Due to its lipophilic characteristic, the complexes formed with mercury and other metals may be redistributed into sensitive cells in the brain following dimercaprol treatment.
Lastest Price from 2,3-Dimercapto-1-propanol manufacturers

US $10.00/KG2025-04-21
- CAS:
- 59-52-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt
US $1.10/g2025-04-17
- CAS:
- 59-52-9
- Min. Order:
- 1g
- Purity:
- 99.0% min
- Supply Ability:
- 100 tons min