Uses and toxicity of ethylene oxide
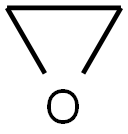
Ethylene oxide, also known as oxane, ethylene oxide, and ETO, it is the simplest cyclic ether and belongs to heterocyclic compounds. Its properties at low temperatures are colorless and transparent liquids (boiling point 10.4℃). ), volatile at room temperature, usually with a pungent odor of ether.
Uses
Ethylene oxide is a strong alkylating agent that can readily react with cellular nucleophiles to inactivate a variety of cellular macromolecules. Ethylene oxide occurs naturally in the body due to its conversion from ethylene, which results from normal metabolic processes and the consumption of plants where it is a natural hormone. Ethylene oxide can also be detected in background air due to the combustion of fossil fuels, its presence in tobacco smoke, and the decay of organic material. These air emission sources are expected to be negligible relative to the fugitive emissions from industrial and medical facilities. As an antimicrobial, ethylene oxide reacts more strongly against bacteria than yeast and fungi. Its sterilant properties are similar to those of heat except that the effects are largely superficial due to limited penetration. Ethylene oxide is typically manufactured by the oxidation of ethylene in the presence of silver catalyst.
Most ethylene oxide (65%) is produced and consumed in the production of ethylene glycol (antifreeze). It is also used in the production of nonionic surfactants, polyester resins, soaps, and detergents as well as specialty solvents. Due to its antimicrobial activity, a small percentage of ethylene oxide (<0.1%) is used as a sterilant for heat-sensitive medical devices and pharmaceuticals as well as for stored foods such as herbs and spices.
Mechanisms of toxicity
The mechanisms of toxicity are not yet understood; however, it is likely that, in general, the toxic effects of ethylene oxide are due to its ability to react with cellular molecules, altering function. Attempts to link the carcinogenicity of ethylene oxide noted in experimental animals to ethylene oxide-induced DNA adducts, including the major adduct formed (N7-hydroxyethyl guanine), have been unsuccessful. Research on the mode of action for ethylene oxide-induced carcinogenicity includes work focused on the potential role of glutathione depletion and the resulting oxidative stress, events that can occur after exposure to high levels of ethylene oxide.
Ethylene oxide released to the environment partitions primarily to the atmosphere due to its high volatility (vapor pressure 146 kPa at 20℃). Although the high water solubility of ethylene oxide suggests it can be extracted from air by rainfall, its rapid volatilization from water (half-life of 1 h) argues against this process being a significant factor in its environmental fate. In the atmosphere, ethylene oxide reacts with hydroxyl radicals resulting in a half-life of 2–5 months. In freshwater, ethylene oxide is hydrolyzed to ethylene glycol (half-life ~ 1 week); in salt water, it is hydrolyzed to ethylene glycol and ethylene chlorohydrin (half-life ~2 weeks). In unacclimated aqueous media, ethylene oxide is also subject to biodegradation with estimated half-lives of 1–6 months (aerobic) and 4–24 months (anaerobic). However, in the presence of activated sludge, ethylene oxide is readily biodegradable. Due to its high volatility and water solubility, ethylene oxide is not expected to persist in soil or sediments. The low log Kow (-0.30) for ethylene oxide indicates little potential for bioaccumulation.
You may like
Related articles And Qustion
Lastest Price from ETHYLENE OXIDE manufacturers

US $1.00/KG2025-04-21
- CAS:
- 75-21-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $20.00-1.00/KG2025-03-07
- CAS:
- 75-21-8
- Min. Order:
- 1KG
- Purity:
- 0.99
- Supply Ability:
- 20 tons