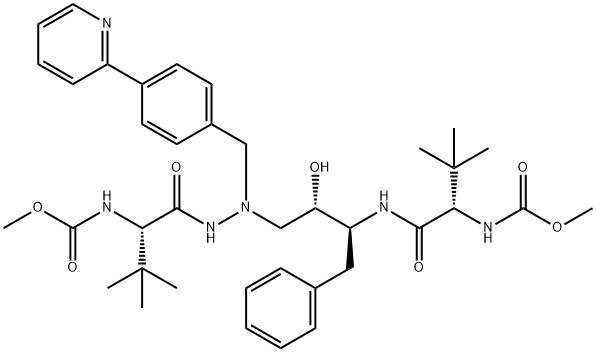
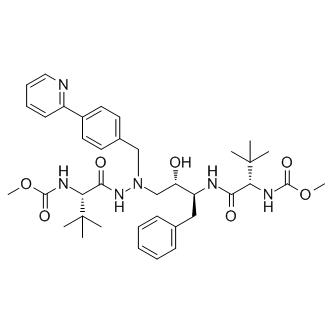
Uses and Side effects of Atazanavir
Atazanavir (ATV) sulfate, (3S,8S,9S,12S)-3,12-bis(1,1-dimethylethyl)- 8-hydroxy-4,11-dioxo-9-(phenylmethyl)-6-[[4-(2-pyridinyl)phenyl] methyl]-2,5,6,10,13-pentaazatetradecanedioic acid dimethyl ester sulfate (1:1), previously known as BMS-232632, is an azapeptide inhibitor of HIV-1 protease. Atazanavir is a white–yellow crystalline powder and slightly soluble in water; the pH of a saturated solution in water is 1.9 (at 24731C). atazanavir (ATV) belongs to the protease inhibitor (PI) class of antiretroviral agents.
Uses
Atazanavir is used in the treatment of HIV. The efficacy of atazanavir has been assessed in a number of well-designed trials in ART-naive and ART-experienced adults.Atazanavir is distinguished from other protease inhibitors in that it has lesser effects on lipid profile and appears to be less likely to cause lipodystrophy. There may be some cross-resistant with other protease inhibitors.When boosted with ritonavir it is equivalent in potency to lopinavir for use in salvage therapy in people with a degree of drug resistance, although boosting with ritonavir reduces the metabolic advantages of atazanavir.
Mechanism of action
The HIV-1 protease, synthesized as part of the Gag–Pol precursor protein, has 99 amino acids and is an aspartic acid protease. It is not active in the monomeric form and requires dimerization in order to attain catalytic activity. The active site of the protease is formed along the dimer interface, with each subunit contributing one of the two catalytic aspartic acid residues to form a nearly symmetrical structure. HIV-1 protease inhibitors were designed to inhibit the wild-type protease by binding to the active site. Inhibition of HIV protease activity results in a failure of HIV virion maturation, as viral Gag and Gag–Pol polyproteins cannot be proteolytically cleaved into individual proteins. HIV virions containing these uncleaved polyproteins ("immature" virions) can bind and enter susceptible cells, but are unable to replicate.
Bioavailability
ATV is rapidly absorbed after oral administration, with a Tmax of 2.5 h. ATV requires acidic gastric pH for dissolution and reduction of the gastric pH severely impacts on ATV bioavailability. For this reason, unboosted ATV cannot be used in patients using PPIs, H2-antagonists, or didanosine (ddI). Food enhances the bioavailability of ATV. Increases in AUC and Cmax when the drug was given with a light meal or high-fat meal were 70% and 57% (light meal) and 35% and no change in Cmax (high fat), respectively,compared with administration when the subject had fasted. The coefficient of variation of these pharmacokinetic (PK) parameters was reduced substantially when the drug was given with food. Mean halflife (t1/2) in healthy volunteers was 7.9 (7s.d. 2.9) h and in HIVinfected adults 6.5 (7s.d. 2.6) h. ATV is 86% plasma protein bound with equal binding to albumin and alpha-1-acid glycoprotein.
Side effects
Common side effects include: nausea, jaundice, rash, headache, abdominal pain, vomiting, insomnia, peripheral neurologic symptoms, dizziness, muscle pain, diarrhea, depression and fever.Bilirubin levels in the blood are normally asymptomatically raised with atazanavir, but can sometimes lead to jaundice.
You may like
Lastest Price from Atazanavir manufacturers

US $0.00/Kg/Bag2025-04-21
- CAS:
- 198904-31-3
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 100KGS

US $0.00-0.00/KG2025-04-15
- CAS:
- 198904-31-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg