Uses and Reaction of Ammonium Carbamate
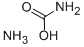
Ammonium carbamate is a white crystalline solid; ammonia odor; very volatile; it is made from liquid NH3 and solid CO2.

Properties
Ammonium carbamate is a white solid that is extremely soluble in water, less so in alcohol. Ammonium carbamate can be formed by the reaction of ammonia NH3 with carbon dioxide CO2, and will slowly decompose to those gases at ordinary temperatures and pressures.
Reaction
In a closed container solid ammonium carbamate is in equilibrium with carbon dioxide and ammonia.
[NH4][NH2CO2] ⇌ 2 NH3 + CO2
Lower temperatures shift the equilibrium towards the carbamate.
At higher temperatures ammonium carbamate condenses into urea:
[NH4][NH2CO2] → (NH2)2CO + H2O
This reaction was first discovered in 1870 by Bassarov, by heating ammonium carbamate in sealed glass tubes at temperatures ranging from 130 to 140 °C.
Uses
Ammonium carbamate is an intermediate in the industrial production of urea. It has also been approved by the Environmental Protection Agency as an inert ingredient present in aluminium phosphide pesticide formulations. This pesticide is commonly used for insect and rodent control in areas where agricultural products are stored.
Ammonium carbamate can be used as a good ammoniating agent, though not nearly as strong as ammonia itself. For instance, it is an effective reagent for preparation of different substituted β-amino-α,β-unsaturated esters. The reaction can be carried out in methanol at room temperature and can be isolated in the absence of water, in high purity and yield.
Ammonium carbamate can be a starting reagent for the production of salts of other cations.
Preparation
Ammonium carbamate can be prepared by reaction of the two gases at high temperature (175–225 °C) and high pressure (150–250 bar).
It can also be obtained by bubbling gaseous CO2 and NH3 in anhydrous ethanol, 1-propanol, or DMF at ambient pressure and 0 °C. The carbamate precipitates and can be separated by simple filtration, and the liquid containing the unreacted ammonia can be returned to the reactor. The absence of water prevents the formation of bicarbonate and carbonate, and no ammonia is lost.
See also
Lastest Price from AMMONIUM CARBAMATE manufacturers

US $0.00-0.00/KG2020-02-26
- CAS:
- 1111-78-0
- Min. Order:
- 1KG
- Purity:
- 99.0%+
- Supply Ability:
- 100 tons

US $1.00/KG2019-07-10
- CAS:
- 1111-78-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100kg