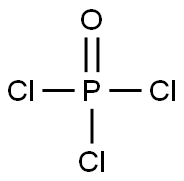
Uses and preparation of Phosphorus oxychloride
Phosphorus oxychloride is an inorganic compound which is commonly referred to as phosphorous oxychloride or as phosphoryl chloride. At room temperature & pressure, this compound is a colourless liquid that forms fumes in moist air. These fumes are released because POCl3 undergoes hydrolysis in the presence of moisture to yield phosphoric acid and hydrogen chloride (which evolves as fumes).

Physical properties
With a freezing point of 1 °C and boiling point of 106 °C, the liquid range of POCl3 is rather similar to water. Also like water, POCl3 autoionizes, owing to the reversible formation of POCl2+,Cl−.
Chemical properties
POCl3 reacts with water to give hydrogen chloride and phosphoric acid:
O=PCl3 + 3 H2O → O=P(OH)3 + 3 HCl
Intermediates in the conversion have been isolated, including pyrophosphoryl chloride, P2O3Cl4.
Upon treatment with excess alcohols and phenols, POCl3 gives phosphate esters:
O=PCl3 + 3 ROH → O=P(OR)3 + 3 HCl
Such reactions are often performed in the presence of an HCl acceptor such as pyridine or an amine.
POCl3 can also act as a Lewis base, forming adducts with a variety of Lewis acids such as titanium tetrachloride:
Cl3PO + TiCl4 → Cl3POTiCl4
The aluminium chloride adduct (POCl3·AlCl3) is quite stable, and so POCl3 can be used to remove AlCl3 from reaction mixtures, for example at the end of a Friedel-Crafts reaction.
POCl3 reacts with hydrogen bromide in the presence of Lewis-acidic catalysts to produce POBr3.
Preparation
Phosphoryl chloride can be prepared by many methods. Phosphoryl chloride was first reported in 1847 by the French chemist Adolphe Wurtz by reacting phosphorus pentachloride with water.
By oxidation
The commercial method involves oxidation of phosphorus trichloride with oxygen:
2 PCl3 + O2 → 2 POCl3
An alternative method involves the oxidation of phosphorus trichloride with potassium chlorate:
3 PCl3 + KClO3 → 3 POCl3 + KCl
Oxygenations
The reaction of phosphorus pentachloride (PCl5) with phosphorus pentoxide (P4O10).
6 PCl5 + P4O10 → 10 POCl3
The reaction can be simplified by chlorinating a mixture of PCl3 and P4O10, generating the PCl5 in situ. The reaction of phosphorus pentachloride with boric acid or oxalic acid:
3 PCl5 + 2 B(OH)3 → 3 POCl3 + B2O3 + 6 HCl
PCl5 + (COOH)2 → POCl3 + CO + CO2 + 2 HCl
Uses
In one commercial application, phosphoryl chloride is used in the manufacture of phosphate esters. Triarylphosphates such as triphenyl phosphate and tricresyl phosphate are used as flame retardants and plasticisers for PVC. Trialkylphosphates such as tributyl phosphate are used as liquid–liquid extraction solvents in nuclear reprocessing and elsewhere.
In the semiconductor industry, POCl3 is used as a safe liquid phosphorus source in diffusion processes. The phosphorus acts as a dopant used to create n-type layers on a silicon wafer.
You may like
Related articles And Qustion
See also
Lastest Price from Phosphorus oxitrichloride manufacturers

US $10.00/KG2025-04-21
- CAS:
- 10025-87-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $30.00/KG2023-10-09
- CAS:
- 10025-87-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20T