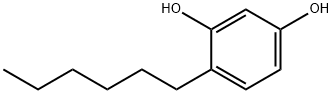
Uses and Characteristics in Cosmetics of Hexylresorcinol
Hexylresorcinol is an organic compound with local anaesthetic, antiseptic, and anthelmintic properties.
It is available for use topically on small skin infections, or as an ingredient in throat lozenges. It is marketed as S.T.37 by Numark Laboratories, Inc. (in a 0.1% solution) for oral pain relief and as an antiseptic. Sytheon Ltd., USA markets hexylresorcinol (trade named Synovea HR). Johnson & Johnson uses hexylresorcinol in its Neutrogena, Aveno, and RoC skincare products as an anti-aging cream. Hexylresorcinol has been used commercially by many cosmetic and personal care companies, such as Mary Kay, Clarins, Unilever, Murad, Facetheory, Arbonne, and many small and large companies. As an anthelmintic, hexyresorcinol was sold under the brand Crystoids.
Melanin and Melanogenesis
Pigmentation
Skin color is one of the most important physical traits of humans because it affects so many aspects of our health and social well-being. Skin color is also one of the best examples of evolution by natural selection acting on the human body. Anthropologic studies have provided us with two important facts: the earliest Homo sapiens had dark skin, rich in protective melanin, and small groups of “modern” humans dispersed out of the African tropics into less intensely sunny parts of Africa, Eurasia, and the Northern Hemisphere. Over time, these latter groups of humans underwent genetic changes leading to the loss of melanin pigmentation.33 Skin color is one of the most conspicuous ways in which humans vary and has been widely used to define human races. Unfortunately, skin color is also a determinant of human interaction and destinies.
Human skin coloration is both adaptive and labile as evidenced by the multitude of different skin colors of humans around the world. Though humans are often characterized as having black, white, red, or yellow skin, no one is actually any of these colors, as these are commonly used terminologies that do not reflect biological reality. Skin coloration in humans arises from a complex series of cellular processes involving the synthesis and transfer of a pigment, melanin, which, besides being responsible for skin color and tone, is the key physiological defense against sun-induced damage, such as sunburn, photo-aging, and photo-carcinogenesis. The melanin itself is formed by a sequence of reactions in which tyrosine is oxidized to dopa and then dopa is subsequently oxidized to melanin. These reactions are catalyzed by the enzyme tyrosinase. The formation of melanin, also known as melanogenesis, is carried out in melanosomes, organelles of that population of cells known as the melanocytes, which are located in the lower part of the epidermis. The melanosomes containing the melanin are subsequently transferred from the melanocytes to the neighboring cells, the keratinocytes, which then transport and distribute the melanin to the upper layers of the skin.
Skin coloration is primarily regulated by the amount and type of melanin synthesized by the melanocytes36,37; however, additional and equally contributing factors include (a) the efficiency of the transfer of the melanosomes, hence the melanin, from the melanocytes to the neighboring keratinocytes, a process that occurs with the help of E-cadherin, an adhesion protein, and (b) the subsequent distribution and degradation of the transferred melanosomes by the recipient keratinocytes. Although a number of factors can influence the formation of melanin, exposure of the skin to UV light markedly influences and increases the amount and rate of melanin production, most often producing a further darkening of the skin or a “tan.” Sun exposure as well as hormones and other environmental and physiological factors can also lead to more localized skin pigmentation, e.g., age spots, melasma, and freckles, owing to an overabundance of melanin production in the afflicted areas of the skin.
Skin Lightening/Even-Toning
For a substantial segment of the human population, one’s natural skin color is not satisfactory and efforts are undertaken to modify it. For example, in North America, Europe, and Australia, many endeavor to enhance skin pigmentation through tanning, whether natural (induced melanin production) or artificial (dyes). Others, especially certain Asian cultures, endeavor to prevent skin coloration and/or seek to actually lighten their natural skin color. Additionally, skin lightening efforts are oftentimes undertaken to address or eliminate age spots, melasma, and freckles, or to obtain even-toning effect with one’s skin. HR has been found to have a significant skin lightening effect due to a strong inhibitory effect on tyrosinase and peroxidase and a stimulatory effect on glutathione and E-cadherin syntheses. It is believed that the HR bind to tyrosinase directly and inhibits its enzyme activity. Additionally, having observed that fragments of DNA can stimulate melanin synthesis, it seems that the reduction of DNA damage by HR, as previously noted, may also be responsible for inhibition of melanin synthesis.
Chen et al. found that HR inhibits both the monophenolase (tyrosine to DOPA) and diphenolase (DOPA to DOPAchrome) activity of mushroom tyrosinase. Its activity or mode of action, as shown by a kinetic analysis, is as a competitive inhibitor. More recently, Chaudhuri demonstrated that HR is a far superior tyrosinase inhibitor (using both tyrosine and DOPA substrates) than the three commercially available skin lighteners: hydroquinone, kojic acid, and licorice extract. Concurrently, HR was found to provide superior inhibition of peroxidase activity of HR as compared to the three skin lighteners tested as well.12 This study also showed that at 10 μg/mL use level, HR had inhibitory effects on extracellular and intracellular melanin production by 75% and 36%, respectively, when compared with placebo. Related studies using B16 melanoma cells showed that their growth rate was not significantly altered in the presence of HR during a 72 h incubation period, indicating that the HR-induced regulatory effects on melanogenesis of melanoma cells occurred without affecting cell proliferation.
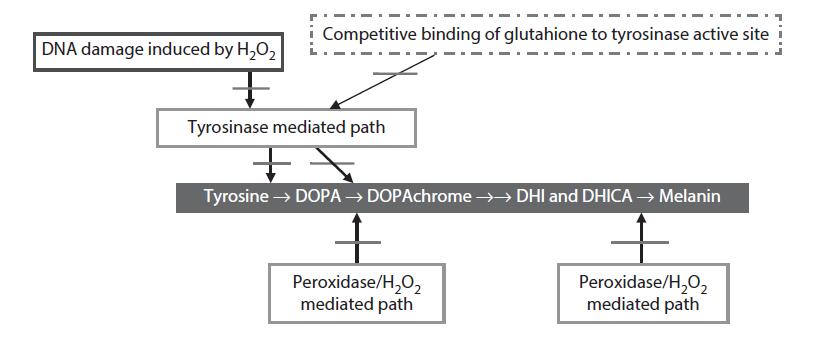
FIGURE 7.2 Sites in the melanogenesis pathway in melanocytes inhibited by HR. DOPA = 3,4-dihydroxyphenylalanine; DOPAchrome = 5,6-dioxo-2,3,5,6-tetrahydro-1H-indole-2-carboxylic acid; DHI = 5,6-dihydroxyindole; DHICA = 5,6- dihydroxyindole-2-carboxylic acid.
Literature data shows that low glutathione levels relates to the deposition of melanin in the skin of humans and other animals, whereas high glutathione levels inhibit melanogenesis. It is also reported that glutathione depletion increases tyrosinase activity in human melanoma cells. UVA irradiation induces the immediate loss of reduced glutathione (GSH) in both melanocytes and keratinocytes. Recently, Matsuki et al. have reported that glutathione has a dose dependent inhibition on melanin synthesis in the reaction of tyrosinase and L-DOPA. Since HR has a dose dependent increase effect on glutathione synthesis, it can be concluded that HR also inhibits melanogenesis by stimulating glutathione synthesis.
It has also been found that human skin exposed to UVB irradiation with a dose of 2 MED manifests a significant increase in the expression of Endothelin-1 (ET-1) and tyrosinase mRNA signals five days after irradiation.45,46 In these studies, low levels of ET-1 secreted by keratinocytes in response to UVR was shown to down-regulate E-cadherin in melanocytic cells. ET-1 is a potent down-regulator of E-cadherin in human melanocytes and also melanoma cells. An independent study by Chaudhuri has shown a 15-fold up-regulation of CDH-1 gene coding for E-cadherin as compared to a control in UVB irradiated normal human keratinocytes treated with HR. In light of the foregoing, it is quite tempting to propose that HR also reduces UV-induced hyperpigmentation by modulating E-cadherin.
Eller et al. have shown that DNA damage after UV irradiation enhances UV-induced melanization. They have also shown that the addition of small DNA fragments to un-irradiated pigment cells in vitro or in vivo to guinea pig skin induces a pigment response indistinguishable from UV-induced tanning. Based on the study reported by Yen et al. which found that HR reduces DNA damage induced by hydrogen peroxide; it is quite conceivable to conclude that HR also inhibits melanin synthesis by reducing DNA damage.
Taken together, the data generated by the present author and available in the literature strongly suggests that HR provides for a reduction in melanin production by modulating multiple sites in the melanogenesis pathway in melanocytes. This pathway and the sites of action of HR (shown by arrows) are portrayed in Figure 7.2.
Human Clinical
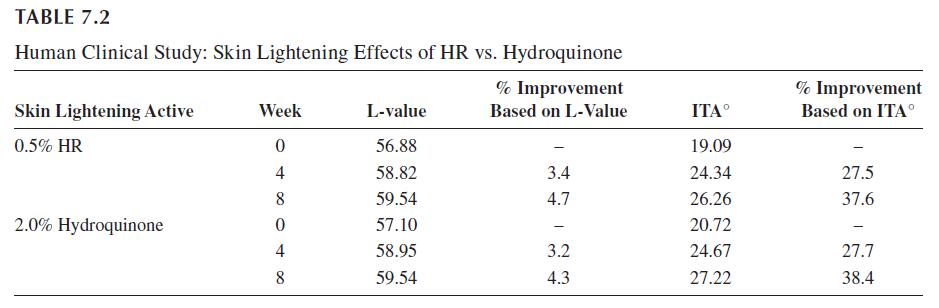
In addition to the multitude of in vitro and non-human in vivo studies on the effects of HR on skin mechanisms and physiology, many of which are discussed above, there are a number of human clinical studies that have been reported. Chaudhuri has reported on the skin-lightening efficacy and safety of a lotion containing 0.5% HR compared with a 2% hydroquinone lotion. This was a single-center study of 15 subjects who applied the lotions twice a day, morning and evening, for eight weeks. The test sites, as well as untreated sites on each forearm, were evaluated for skin color change as represented by the change in L values and ITA° (Individual Topology Angle—COLIPA SPF test method). Changes were measured by chromometric measurement. The results of this study are presented in Table 7.2 where the delta represents the percent change in skin color from the baseline coloration of the untreated skin. According to these results, the HR lotion was found to provide a comparable, statistically significant (p value ≥ 0.001) change in skin color to that attained with the like composition containing 2% hydroquinone. Such results are consistent with a “lightening” of the skin. No adverse effects were noted for either lotion over the test period.
Another clinical study was carried out by the present author using 1% HR lotion for treating individuals having hyper-pigmented spots. Eighteen volunteers between the ages of 42 and 60, of which there was a mix of Caucasian (10), Asian (7), and Hispanic (1), applied the 1% HR lotion twice daily in an amount sufficient to cover their upper arm, including specifically the targeted hyper-pigmented spots. The treated skin of each volunteer was evaluated and the percent reduction in pigmentation of the treated skin was determined using ITA degree. When compared to the coloration on day zero (pre-application), it was found that the treated hyper-pigmented spots showed a statistically significant reduction of 39% and 60% in pigmentation at four and eight weeks, respectively. On the other hand, the treated area of the skin surrounding the hyper-pigmented spots showed only marginal changes when compared to day zero (pre-application). Based on this study, one could conclude that a 1% HR lotion provides an even-toning effect.
Chaudhuri also investigated the potential for HR to show a synergistic effect when used in combination
with other skin lightening ingredients. In this study, a lotion containing 2% by weight of a
commercial blend of HR (25%) and ethyl linoleate (EL, 25%) in caprylic/capric triglycerides (trade
name Asyntra® SL) was applied twice daily for a period of four weeks to the whole face of 15 volunteers.
As in the previous studies, skin color was determined by ITA degrees before and after the treatment.
The results of this study demonstrated a statistically significant (p ≤ 0.05) lightening effect on
the natural skin color (initial ITA degree 2.1 vs. 5.8 after four weeks of treatment). The combination
was also found to be much more effective than HR alone in lightening one’s natural skin color. The
synergy of the ingredients of this blend was subsequently confirmed by carrying out an in vitro study
using B16 melanoma cells. In that study, referenced in the preceding section, an almost identical level
of reduction in melanin synthesis was observed using the HR-EL blend (contains only 25% HR) as
compared to the use of HR alone. Although the full mechanism is unknown, it has been reported that
EL is hydrolyzed to linoleic acid (LA) in vivo, which has been reported to degrade post-translational
tyrosinase and is also involved in the increase in cell turnover. Therefore, it can be presumed that, in
addition to the synergistic effect with HR, EL is also providing all of the skin benefits resulting from
its conversion to LA.
Independently, Makino et al. reported on a single-center, double-blind comparison clinical study of
18 subjects in which the efficacy of a HR-containing (included several other skin lightening ingredients)
cream for reducing ultraviolet-induced hyperpigmentation was evaluated. Test sites were irradiated
with 1.0, 1.5, 2.0, and 2.5 minimal erythema doses. After five days during which pigmentation was
allowed to develop, the HR-containing product or a 4% hydroquinone cream was applied to the respective
test sites, once daily for four weeks. Chroma meter measurements (L* brightness) and standardized digital photographs were taken of the test sites twice a week. According to their findings, areas treated
with the HR-containing product produced greater increases in L* brightness as compared with those
treated with the 4% hydroquinone cream. This study demonstrates that a product designed to affect
multiple pathways of melanogenesis and melanin distribution may provide an additional treatment option
beyond hydroquinone for hyperpigmentation.
Formulation
Having demonstrated the benefits of HR as a skin treatment, HR also benefits from an ease of use in terms of formulating products, especially skin care and cosmetic products. This is especially so at use levels of 0.5%–1.0% (w/w) of HR in the finished formulation, which have been found to provide effective products.
HR is highly soluble (>20%) in a number of cosmetic and skin care ingredients and carriers including caprylic/capric triglycerides, isosorbide dicapylate, ethyl linoleate, ethoxydiglycol, and other high polarity esters; non-ionic solubilizers; glycerol; and a wide range of glycols. PEG-400 was found to be an excellent solubilizer of HR (>50%). Such solubility provides a wide range of options to develop elegant formulations with HR. HR can also be easily combined with other skin lightening ingredients, such as niacinamide, ethyl linoleate, acetyl-glucosamine, ascorbyl-2-glucoside (not magnesium and sodium ascorbyl phosphate as these two requires slightly basic formulation pH), standardized plant extracts, such as Phyllathus emblica (trade named Emblica®), Terminalia chebula (trade named Synastol® TC), etc., thereby opening the door to a multitude of synergistic products to make HR even more effective.
Generally, the pH of the formulation to which HR is added must be acidic due to the presence of phenolic OH in HR. Similarly, it is found that the use of non-ionic emulsifiers is preferable to anionic types. For the preparation of serum or transparent gels, the use of non-ionic solubilizers having high HLB values like PEG-40 hydrogenated castor oil, Laureth 23, Polysorbate 20, and Polysorbate 80 are recommended.
Finally, the addition of HR to various formulations may cause a drop in overall viscosity of the product. In such cases, anionic (such as Xanthan gum, Carbomers) or neutral thickeners (such as Cellulosics) can be added for maintaining and/or restoring the desired viscosity. If needed, low levels 0.1%–0.2% of propyl gallate (SynoxylÒ PGL) can be used to minimize color shift over time.
REFERENCES
10. Ross AB, Kamal-Eldin A, Aman PD. Alkylresorcinols: Absorption, bioactivities, and possible use as biomarkers of whole-grain. Wheat- and rye-rich foods. Nutr Rev 2004;62(3):81–95, and references cited therein.
11. Kozubek A, Tyman JHP. Resorcinolic lipids, the natural nonisoprenoid phenolic amphiphiles and their biological activity. Chem Rev 1999;99:1–25.
12. Chaudhuri RK. Effective skin lightening with protective property. Personal Care 2010;39–44. 13. Fed. Reg. 1991;56(140):33644.
14. Taylor JA, Mitchenall LA, Rejzek M et al. Application of a novel microtitre plate assay for the discovery of new inhibitors of DNA gyrase and DNA topoisomerase VI. PLoS One 2013;8(2):e58010.
15. Payne DJ, Gwynn MN, Holmes DJ et al. Drugs for bad bugs: Confronting the challenges of antibacterial discovery. Nat Rev Drug Discov 2007;6:29–40.
16. Wong CF, Barnes LM, Dahler AL et al. E2F suppression and Sp1 over expression are sufficient to induce the differentiation-specific marker, transglutaminase type 1, in a squamous cell carcinoma cell line. Oncogene 2005:24:3525–34.
17. Kim SG, Kim AS, Jeong JH et al. 4-Hexylresorcinol stimulates the differentiation of SCC-9 cells through the suppression of E2F2, E2F3 and Sp3 expression and the promotion of Sp1 expression. Oncol Rep 2012;28:677–81.
18. Kim SG, Choi JY. 4-Hexylresorcinol exerts antitumor effects via suppression of calcium oscillation and its antitumor effects are inhibited by calcium channel blockers. Oncol Rep 2013;29(5):1835–40.
19. Mangala LS, Fok JY, Zorrilla-Calancha IR et al. Tissue transglutaminase expression promotes cell attachment, invasion and survival in breast cancer cells. Oncogene 2006;26:2459–70.
20. Fok JY, Ekmekcioglu S, Mehta K. Implications of tissue transglutaminase expression in malignant melanoma. Mol Cancer Ther 2006:5:1493–503.
You may like
Related articles And Qustion
See also
Lastest Price from 4-Hexylresorcinol manufacturers
US $0.00-0.00/kg2025-09-08
- CAS:
- 136-77-6
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1

US $1000.00-700.00/ton2025-04-21
- CAS:
- 136-77-6
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 5000