Use of Diflubenzuron
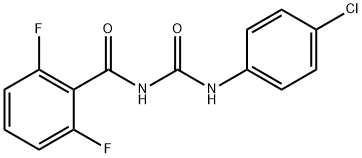
Diflubenzuron is produced by the reaction of 2,6-difluorobenzamide with 4-chlorophenylisocyanate. Diflubenzuron was first registered as a pesticide in the United States in 1979. Environmental Protection Agency issued a Registration Standard for diflubenzuron in September 1985 (aPB86-176500). In 1991, a Data Call-In was made to require additional residue chemistry and ecological effects data. The current Reregistration Eligibility Decision Document was issued in August 1997, reflecting analysis of the new data.

Uses
Diflubenzuron is currently registered for use on cotton, citrus, mushrooms, pastures, soybeans, and ornamentals. It is also used in forestry, cattle, and in public health program. No residential usage is registered. Its target species include many leaf eating insects, such as grasshoppers, gypsy moth, forest tent caterpillar, Nantucket pine tip moth, velvet bean caterpillar, green cloverworm, beet armyworm, Mexican bean beetle, mosquito larvae, aquatic midge, rust mite, boll weevil, citrus root weevil, West Indian sugarcane rootstalk borer/weevil, sciarid fly, and face fly. Formulations of diflubenzuron include solution, suspension concentrate, flowable concentrate, bolus, granules, and bait stations. Diflubenzuron is applied by airblast, aircraft, and hydraulic sprayers, bait stations, and internal bolus.
Environmental Fate
Diflubenzuron is an odorless, white, crystalline solid with a melting point of 230–232 ℃. It is almost insoluble in water (0.2 mg l-1) and poorly soluble in apolar organic solvents.It is almost nonvolatile. It is relatively stable in acidic and neutral media but hydrolyses under alkaline conditions.Diflubenzuron is difficult to be degraded in sterilized water under neutral or acidic conditions. However, it is degraded rapidly under field conditions. Application of diflubenzuron to water resulted rapid partition to sediment; the parent compound and 4-chlorophenylurea (CPU) may persist on sediment for more than 30 days.
The rate of degradation of diflubenzuron in soil is strongly
dependent on the particle size. For larger particles
(10 microns), the half-life is 8–16 weeks and for smaller
particles (2 microns), it is 0.5–1 week. Almost all of the parent
compound breaks down to form 2,6-difluorobenzoic acid
(DFBA) and CPU. A very minor amount forms 4-chloroaniline
(PCA) which rapidly binds to the soil. Under field conditions,
diflubenzuron has very low mobility.
Very little diflubenzuron is absorbed, metabolized, or
translocated in plants. It also is not readily taken up from
treated soil.
Diflubenzuron has very low vapor pressure (<2×10-7 Pa
at 25°C) and its atmospheric half-life is only several hours.
Therefore, it is not expected that diflubenzuron will be present
in air for extended periods and the long-range transport and
redeposition of diflubenzuron is expected to be negligible.
Mechanism of Toxicity
Diflubenzuron inhibits the enzyme chitin synthase, which is required in the final step of chitin synthesis. Chitin is a polysaccharide and a major constituent of the exoskeleton of insects. In insects, the trachea is held open by rings of chitin. The exoskeleton and waxy covering also prevent water loss. Inhibiting chitin synthesis therefore can provide an effective means of pest control. Vertebrates and most plants do not utilize chitin, thus making diflubenzuron a target-selective pesticide.
After repeated dosing of diflubenzuron, 4-chloroaniline concentration in erythrocyte can arrive at 392 ng g-1 which may explain the occurrence of methemoglobinemia. Other toxicological mechanisms may include increasing of the activity of liver enzymes, increasing of organ weights, and histopathological changes including hemosiderosis and mild cell necrosis.
You may like
Related articles And Qustion
Lastest Price from Diflubenzuron manufacturers

US $120.00-65.00/kg2025-04-21
- CAS:
- 35367-38-5
- Min. Order:
- 1kg
- Purity:
- 97%
- Supply Ability:
- 20MT

US $10.00/KG2025-04-21
- CAS:
- 35367-38-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt