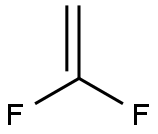
Preparation of 1,1-Difluoroethylene
1,1-Difluoroethylene is used in the manufacture of polyvinylidene fluoride for its use as a thermal, chemical, and ultraviolet light–resistant agent, and as an anticorrosive agent. The monofilament form is used as filter cloth in the pulp and paper industry. Due to its high melting temperature, it can be used as an insulator. Some of its copolymers are used for their heat- and moisture-resistant properties, primarily in industrial, aerospace, and automotive applications.
Preparation
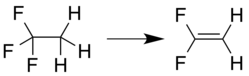
1,1-Difluoroethylene can be prepared by elimination reaction from a 1,1,1-trihaloethane compound, for example, loss of hydrogen chloride from 1-chloro-1,1-difluoroethane:.

or loss of hydrogen fluoride from 1,1,1-trifluoroethane:
Environmental Fate
1,1-Difluoroethylene is a colorless gas. It is slightly soluble in
water, but soluble in organic solvents such as alcohol and
ether. Its boiling point is -85°C, melting point is -144°C,
specific gravity is 0.617 g cm-3, and vapor pressure is
3.0×104 mm Hg. Its octane/water partition coefficient is 1.24
and Henry’s law constant is 0.4 atm-m3 mol-1.
Production and use of 1,1-Difluoroethylene result in its
release to the environment through various waste streams. It
has medium mobility in soil and degrades in soil. It volatilizes
from the contaminated water. Aquatic bioconcentration and
adsorption to sediment are not very significant. As a gas form in
the atmosphere, it reacts with photochemically produced
hydroxyl radicals and degrades.
Metabolism
Oxidative metabolism of 1,1-Difluoroethylene is mediated by the hepatic microsomal monooxygenase to form epoxide and the inactivation of hemeprotein.