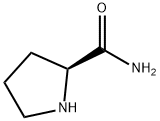
Unraveling the Catalytic Potential of L - Prolinamide in Aldol and Related Reactions
L-Prolinamide is a tripeptide component of thyrotropin-releasing hormone that plays a role in promoting secretion of TSH and prolactin in specific cells. Aside from its well-known endocrine role in the thyroid system, TRH receptors are also thought to act as modulatory neuropeptides in the central nervous system. The cloning of a second receptor for TRH from rat brain and spinal cord provided a possible explanation for certain neurotransmitter actions of TRH, in particular the nociceptive and spinal cord regenerative actions.

Enantioselective direct aldol reactions catalyzed by l-prolinamide derivatives
L-Prolinamides, prepared from l-proline and simple aliphatic and aromatic amines, have been found to be active catalysts for the direct aldol reaction of 4-nitrobenzaldehyde with neat acetone at room temperature. They give moderate enantioselectivities of up to 46% enantiomeric excess (ee). The enantioselectivity increases as the amide N—H becomes a better hydrogen bond donor. l-Prolinamides 3, derived from the reaction of l-proline with α,β-hydroxyamines such that there is a terminal hydroxyl group, show more efficient catalysis and higher enantioselectivities. In particular, catalyst 3h, prepared from l-proline and (1S,2S)-diphenyl-2-aminoethanol, exhibits high enantioselectivities of up to 93% ee for aromatic aldehydes and up to >99% ee for aliphatic aldehydes under –25°C. Model reactions of benzaldehyde with three enamines derived from the condensation of prolinamides with acetone have been studied by quantum mechanics calculations. The calculations reveal that the amide N—H and the terminal hydroxyl groups form hydrogen bonds with the benzaldehyde substrate. These hydrogen bonds reduce the activation energy and cause high enantioselectivity. Our results suggest a new strategy in the design of new organic catalysts for direct asymmetric aldol reactions and related transformations.[1]
The relationship between l-prolinamide structures and the enantioselectivity and theoretical studies on the transition structure and reaction mechanism are presented. The key factors that control the enantioselectivity of the direct aldol reaction catalyzed by l-prolinamides is also clarified. It has been reported that the acidic proton of proline is critical for the reactivity and stereoselectivity of the proline-catalyzed direct aldol reaction. l-Prolinamide (2-pyrrolidine-carboxamide was initially observed to be ineffective in catalyzing the direct aldol reaction. However, we found that in the presence of 20 mol% l-prolinamide the model reaction of 4-nitrobenzaldehyde with neat acetone proceeded cleanly to give in 80% yield with 30% ee. This encouraged us to investigate the catalytic activity of a family of l-prolinamides. Most of l-prolinamides exhibit high catalytic activity for the reaction. The secondary amides with N-alkyl groups show low enantioselectivities of 15–23% ee, which might have resulted from a very weak hydrogen bond formed between the proton of amide groups of these compounds and the aldehyde. However, the secondary amides with N-aryl groups show moderate stereoselectivities of 31–46% ee. In particular, the enantioselectivity increases as the aryl substituent varies from electron-donating to electron-withdrawing, which makes the N—H more acidic and thus a better hydrogen bond donor. It can thus be concluded that the amide group of these catalysts is directly involved in catalysis through hydrogen bonding with the aldehyde substrate.
Environmental Modulation of L-Prolinamide Catalysts
Synthetic chiral catalysts generally rely on proximal functional groups or ligands for chiral induction. Enzymes often employ environmental chirality to achieve stereoselectivity. Environmentally controlled catalysis has benefits such as size and shape selectivity but is underexplored by chemists. We here report molecularly imprinted nanoparticles (MINPs) that utilized their environmental chirality to either augment or reverse the intrinsic selectivity of a chiral l-Prolinamide cofactor. In principle, an anti-product can be produced if the more stable s-trans conformer of the enamine intermediate is shifted to the less stable s-cis. In the literature, such a conformational change is achieved through modifying the l-Prolinamide catalyst to destabilize the s-trans conformer. Our hypothesis is that a suitable microenvironment can also shift the conformational equilibrium, without changing the catalyst. After all, conformation of a molecule is highly dependent on its environment, as amply demonstrated by proteins whose secondary and tertiary structures are strongly impacted by solvents, microenvironments, and also binding partners.[2]
Molecular imprinting is a powerful method to create chiral binding sites. To create a microenvironment to modulate the conformation of a l-Prolinamide catalyst (and ultimately to control its stereoselectivity), we designed template molecule (S,S)-1 for the conjugate addition between aldehyde and (E)-1-nitro-2-phenylethene or β-nitrostyrene. This template molecule has two key units, as shown: the green-colored moiety is used to create a catalyst-binding pocket through molecular imprinting (i.e., the primary chiral site for the l-Prolinamide catalyst); the magenta-colored substructure is the space-holder for a secondary chiral site, which will be used to accommodate the (white-colored) aldehyde and the (magenta-colored) nitroalkene during the catalysis. The l-Prolinamide catalyst is expected to react with the aldehyde to afford an enamine intermediate. Proximity of the enamine and β-nitrostyrene should help their conjugate addition but the resulting ionic intermediate (i.e., iminium cation) is unlikely to prefer the overall hydrophobic active site that has been imprinted against a neutral, nonpolar template.
Preparation of l-Prolinamide with adamantane for aldol reaction catalysis in brine
L-Prolinamide with double-H potential were prepared and employed as organocatalysts in asymmetric aldol reactions. The catalyst with adamantane showed improved catalytic activity, which was further enhanced by using brine as the solvent. A series of aldol reactions in brine at 0 °C provided good yields (up to 98%) with high diastereoselectivities (>99 : 1) and enantioselectivities (>99%). The prepared catalyst was adsorbed by a nanofibrous film of poly(AN-MA-β-CD) via host–guest interaction in the reaction system. The catalyst was separated from the film by applying ultrasound, with a total recovery of 96.2%. The catalyst was reused up to five times without a significant change in diastereoselectivity and enantioselectivity.[3]
L-Prolinamide with adamantane catalyzed the aldol reaction. The reaction of cyclohexanone with m-nitrobenzaldehyde assessed recyclability of catalyst. After run, the catalyst was adsorbed with nanofibrous of polymer via host–guest interaction. In the l-proline-catalyzed reaction, the necessity of using the carboxylic acid of proline remains unclear. Since Tang and co-workers reported l-Prolinamide -catalyzed aldol reactions of 4-nitrobenzaldehyde with acetone, effective organocatalysts have been developed based on proline to afford facile atom economic access to optically pure compounds. Yang et al. used benzyl chloride instead of nitrobenzene sulfonic acid to synthesize prolinamide catalysts, which resulted in faster reactions with good diastereoselectivity in brine at room temperature.
Initially, we employed chiral l-Prolinamide catalysts 1a–1d to screen the direct aldol reaction of p-nitrobenzaldehyde with cyclohexanone at room temperature. A series of experiments were then performed under different reaction conditions (different catalysts, loading, and solvents). In the beginning of the experiment, the reactions were catalyzed by different prolinamides (10 mol%) at room temperature for 36 h in DCM. Interestingly, all catalysts provided the desired aldol products with good yields. The aldol reactions in the presence of l-Prolinamide 1a and 1b generated 69% and 77% of enantioselectivity. When used to catalyze the aldol reaction of 4-nitrobenzaldehyde and cyclohexanone, compounds 1c and 1d afforded good yields with higher diastereoselectivity and enantioselectivity than 1a and 1b. This result may be due to replacement of benzoic acid with the large steric hindrance of adamantane. In summary, organocatalysts based on l-proline or trans-4-hydroxy-l-proline with double-H potential were synthesized. Catalyst 1d (10 mol%) demonstrated very efficient performance for catalyzing direct aldol reactions.
References
[1]Tang Z, Jiang F, Cui X, Gong LZ, Mi AQ, Jiang YZ, Wu YD. Enantioselective direct aldol reactions catalyzed by L-prolinamide derivatives. Proc Natl Acad Sci U S A. 2004 Apr 20;101(16):5755-60.
[2]Li X, Zhao Y. Environmental Modulation of Chiral Prolinamide Catalysts for Stereodivergent Conjugate Addition. J Catal. 2022 Feb;406:126-133.
[3]Wang R, Xu E, Su Z, Duan H, Wang J, Xue L, Lin Y, Li Y, Wei Z, Yang Q. Preparation of prolinamide with adamantane for aldol reaction catalysis in brine and separation using a poly(AN-MA-β-CD) nanofibrous film via host-guest interaction. RSC Adv. 2018 Aug 7;8(50):28376-28385.
You may like
See also
Lastest Price from L-Prolinamide manufacturers

US $0.00/Kg/Drum2025-04-21
- CAS:
- 7531-52-4
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 500kg

US $30.00/kg2025-04-21
- CAS:
- 7531-52-4
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 100 tons