Tungsten Crystal
Tungsten W does not react with oxygen, hydrogen, sulfur, and phosphor directly. It also does not react with acid, aqua regia, and alkali solution. It is slowly soluble in the mixed solution of conc. nitric acid and hydrogen fluoride. W2O3 is formed by heating W in air. W is oxidized on its surface at low vacuum to form WO3 or WO2. These oxides are easy to evaporate, resulting forWwire to thin slowly.
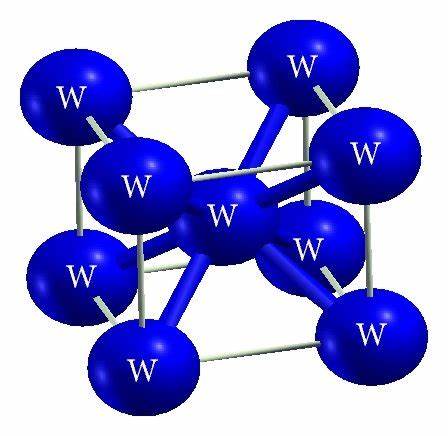
Crystal System
The space lattice of Tungsten W belongs to the cubic system, and its body-centered cubic lattice has a lattice constant of a=0.3158 nm.
Production
W is obtained by oxidizing (Fe, Mn) WO4 or CaWO4 and reducing by the thermit process or by using carbon or hydrogen. Thin films are deposited by vacuum evaporation using the electron beam. The evaporation rate is 1.43×10-4g/cm2 sec at 3309℃.
You may like
Related articles And Qustion
See also
Lastest Price from Tungsten manufacturers

US $6.00/kg2025-04-21
- CAS:
- 7440-33-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month

US $6.00/KG2025-03-28
- CAS:
- 7440-33-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20TONS




