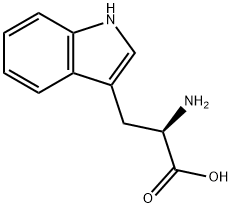
Tryptophan, D(+)-Tryptophan, and L-Tryptophan
Tryptophan
Tryptophan is an essential amino acid used to make proteins. It can not be made in vivo. Nevertheless, Tryptophan is an amino acid needed for normal infant growth and the production and maintenance of the body's proteins, muscles, enzymes, and neurotransmitters. Hence, humans need to get it from their diet. Foods that contain Tryptophan include animal products like chicken and fish and plant foods like nuts or soy[1].
D(+)-Tryptophan and L-Tryptophan
There are two types of Tryptophan: L-tryptophan and D(+)-Tryptophan. D(+)-Tryptophan is not a proteogenic amino acid, while L-tryptophan is a proteogenic amino acid.
L-tryptophan is the L-stereoisomer of Tryptophan. It is an α-amino acid used in protein synthesis. Therefore, L-tryptophan is a proteogenic amino acid. L-tryptophan contains an α-carboxylic acid group, α-amino group, and an indole side chain. Due to an indole side chain, L-tryptophan is a polar molecule with a nonpolar aromatic beta-carbon substituent.
D(+)-Tryptophan is a D-stereoisomer of Tryptophan. This compound occasionally occurs in many peptides, for example, the marine venom peptide contryphan. Another importance of D-tryptophan is the presence of an indole side group. Both serve the same general functions. The only difference between the two types is the orientation of the molecule.

The uses of D-tryptophan
D(+)-Tryptophan is a non-protein amino acid widely used in food and agriculture. Although it does not participate in protein synthesis, D-tryptophan shows special physiological functions. D-amino acid synthesis is a strategy for adapting to changing environmental conditions in bacteria. The inhibitory effects of D-amino acids, especially D-tryptophan, on biofilm development have been reported in foodborne microorganisms such as Pseudomonas aeruginosa, Bacillus subtilis, and Staphylococcus aureus. Further analysis indicated that the inhibitory effects of D-tryptophan on biofilm development might be attributed to changes in initial adhesion between cells and the properties of the extracellular matrix. Hence, D(+)-Tryptophan may be used as a novel preservative for controlling bacterial growth in foods, particularly in seafood, because of its high effectiveness in environments with high sodium chloride concentrations[2-3].
Wang et al. found that D(+)-Tryptophan promoted the production of TGF-β1 cytokines, thereby activating the TGF-β signaling pathway, regulating the downstream target gene expression, and finally resulting in the physiological events, especially Epithelial-mesenchymal transition. Their study provided D(+)-Tryptophan as a potential approach for cancer treatment, wound healing, organ development, and other relevant applications.
References
[1] Kang Xu. "Redox Properties of Tryptophan Metabolism and the Concept of Tryptophan Use in Pregnancy." International Journal of Molecular Sciences 18 7 (2017).
[2] Du, Gengyu et al. "Fluorescent recognition of l- and d-tryptophan in water by micelle probes†." Materials Chemistry Frontiers 8 (2020): 2384–2388.
[3] Chong Wang. "D-tryptophan triggered epithelial-mesenchymal transition by activating TGF-β signaling pathway." Food Science and Human Wellness 11 5 (2022): Pages 1215-1221.
You may like
Related articles And Qustion
Lastest Price from D-Tryptophan manufacturers

US $0.00/kg2025-07-18
- CAS:
- 153-94-6
- Min. Order:
- 1kg
- Purity:
- 98%min
- Supply Ability:
- 500kgs

US $0.00/g2025-06-06
- CAS:
- 153-94-6
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 20000tons