Trospium Chloride: Therapeutic Efficacy, Dosage and Administration
General Description
Trospium chloride, a medication used for overactive bladder, has demonstrated therapeutic efficacy in numerous clinical trials. At a standard dosage of 20mg twice daily, Trospium chloride consistently reduces urinary frequency, urge incontinence episodes, and urgency severity scores while improving voided volume. Studies show significant improvements compared to placebo, with early effects seen within a week. Trospium chloride enhances quality of life and urodynamic parameters, such as increased bladder capacity. Even lower doses have shown positive outcomes, especially in pediatric patients. Dosage adjustments are necessary for specific populations, and caution is advised for certain conditions. Understanding its anticholinergic effects on medication absorption and potential interactions is crucial for safe use. Overall, trospium chloride is a valuable option for managing overactive bladder symptoms, supported by robust clinical evidence.

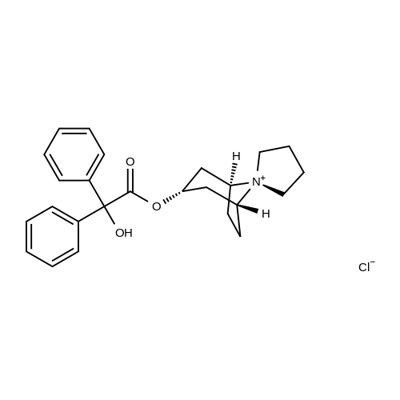
Figure 1. Trospium chloride
Therapeutic Efficacy
Overactive bladder is a highly prevalent, debilitating condition which is associated with considerable frustration on the part of the patient, as well as deterioration in HR-QOL. The condition is also a major economic burden for patients, caregivers, healthcare providers and society as a whole.
Antimuscarinic agents have historically formed the mainstay of overactive bladder management. However, many of these agents lack selectivity for the bladder and can have widespread systemic anti-cholinergic adverse effects. Trospium chloride represents an important addition to the range of available pharmacological treatments of overactive bladder, as it has efficacy similar to that of other antimuscarinic agents but is better tolerated, especially compared with the frequently prescribed oxybutynin.
However, to clarify whether trospium chloride is potentially superior in terms of tolerability compared with several of its comparators, not just oxybutynin, specific and well designed studies need to be conducted in the appropriate clinical setting. This also applies to the drug interaction potential of trospium chloride relative to that of other antimuscarinic agents, as pharmacokinetic studies have suggested that trospium chloride may have a lower potential for clinically relevant drug interactions than other antimuscarinic agents.
Substantial improvements in HR-QOL, efficacy in the elderly and major cost savings regarding overall incontinence management in patients with overactive bladder suggested by post-marketing surveillance studies warrant further investigation in well designed clinical trials. Several other aspects of the clinical profile of trospium chloride would also benefit from additional study. While not currently recommended for use in children, preliminary studies have indicated that trospium chloride may be useful in this population. The potential clinical utility of intravesical trospium chloride, especially in patients with bladder spasms resulting from indwelling catheters, also requires further evaluation. Another interesting avenue of research may be to explore the potential clinical benefit of combining trospium chloride with other therapies.1
Dosage and Administration
Trospium chloride is prescribed for adults with detrusor instability or hyper-reflexia and associated symptoms like urinary frequency, urgency, and urge incontinence. The recommended dosage is 20mg taken twice daily before meals. In certain cases, such as in patients aged 75 and above experiencing anticholinergic side effects or those with severe renal impairment, the dosage may need to be reduced to 20mg once daily. This medication is not suitable for children under 12 years old and should be avoided in individuals with conditions like urinary retention, gastric retention, uncontrolled narrow-angle glaucoma, or those at risk of these issues. Patients with bladder outflow obstruction, ulcerative colitis, intestinal atony, myasthenia gravis, moderate or severe hepatic impairment, as well as pregnant or breastfeeding women, should use trospium chloride with caution. Due to its anticholinergic properties impacting gastric motility, the absorption of other medications may be affected when taken alongside trospium chloride. Furthermore, combining it with other anticholinergic agents can potentially intensify common side effects like dry mouth or constipation. It is essential for healthcare providers to consider these factors when prescribing trospium chloride to ensure safe and effective treatment. 2
Reference
1. Rovner ES. Trospium chloride in the management of overactive bladder. Drugs. 2004;64(21):2433-2446.
2. Sanctura™ (trospium chloride) 20mg tablets [prescribing information]. 2004; Troisdorf: Maduas AG.
You may like
Related articles And Qustion
See also
Lastest Price from Trospium chloride manufacturers

US $0.00-0.00/KG2025-04-21
- CAS:
- 10405-02-4
- Min. Order:
- 1KG
- Purity:
- 99%-101%;BP
- Supply Ability:
- 100 kgs
US $0.00/KG2024-09-25
- CAS:
- 10405-02-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 TONS