Tris Hydrochloride: Preparation Method, Precautions, Advantages and Limitations
General Description
Tris Hydrochloride buffer solution is commonly used in biochemical research. Preparation methods include mixing Tris and HCl solutions or dissolving Tris base and adding HCl dropwise. Precautions involve controlling pH variations, managing reactivity with certain molecules, preventing CO2 absorption, and using compatible electrodes. Tris Hydrochloride has advantages like versatile pH range and minimal interference with biochemical processes but limitations such as pH sensitivity to concentration and temperature, CO2 absorption, and interference with pH electrodes. Despite limitations, Tris Hydrochloride remains popular for its versatility and compatibility with biological systems, making it a preferred choice for buffering applications in laboratories.

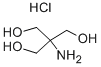
Figure 1. Tris Hydrochloride
Preparation Method
Tris Hydrochloride, commonly known as Tris-HCl, is a buffer solution widely used in biochemical and molecular biology research. There are two main methods for preparing Tris Hydrochloride buffer solution. The first method involves separately preparing 0.05 mol/L Tris and 0.05 mol/L HCl solutions, then mixing them in the volumes specified in commonly used tables. However, due to the difficulty in preparing standard concentrations of dilute hydrochloric acid, another commonly used method is preferred. For example, to prepare 1 L of 0.1 mol/L Tris Hydrochloride buffer solution: dissolve 12.11 g of Tris base in 950 mL to 970 mL of deionized water while stirring, then slowly add 4 N HCl dropwise while monitoring the pH using a pH meter until the desired pH is reached. Finally, adjust the volume to 1 L by adding water. This method ensures the accurate preparation of Tris Hydrochloride buffer solution with the desired pH, making it suitable for various biochemical and molecular biology applications. 1
Precautions
When utilizing Tris Hydrochloride buffer, several key precautions must be observed to ensure its effective and safe use in laboratory applications. Firstly, pH variation is a critical consideration. The pH of Tris buffer can be significantly influenced by factors such as temperature and concentration. Therefore, it is essential to prepare the buffer in a controlled environment to maintain consistent pH levels. Secondly, Tris exhibits reactivity with various molecules, including RNAase inhibitors, aldehydes, enzymes, DNA, and certain metals like Cr3+, Fe3+, Ni2+, Co2+, and Cu2+. This reactivity can either enhance or inhibit reactions, depending on the specific system, necessitating careful consideration when using Tris in experimental setups. Thirdly, Tris readily absorbs CO2 from the atmosphere, emphasizing the importance of tightly sealing prepared buffer solutions to prevent contamination and maintain their efficacy. Moreover, Tris can interfere with some pH electrodes, necessitating the use of compatible electrodes with Tris buffer solutions to ensure accurate pH measurements. Furthermore, Tris is not suitable for use in the bicinchoninic acid (BCA) assay, and its hazardous properties require operators to wear gloves and safety goggles when handling Tris to avoid ingestion, inhalation, or skin absorption-related risks. In conclusion, these precautions, including pH control, reactivity management, CO2 absorption prevention, electrode compatibility, avoidance in specific assays, and safety gear usage, are paramount to guarantee the precise and safe utilization of Tris Hydrochloride buffer in laboratory settings. 2
Advantages and Limitations
Tris Hydrochloride is a versatile buffering agent used in various biochemical and molecular biology applications. Its advantages stem from its strong alkalinity, allowing for the preparation of a wide pH range (from acidic to alkaline) buffering systems using only Tris as the buffering agent. Additionally, Tris Hydrochloride has minimal interference with biochemical processes, as it does not precipitate with calcium, magnesium ions, or heavy metal ions. However, Tris buffering systems also have several drawbacks. Firstly, the pH of the buffer is significantly affected by the concentration of the solution, with a tenfold dilution resulting in a pH change greater than 0.1. Secondly, Tris buffers exhibit a significant temperature dependency, with a temperature coefficient (ΔpKa/°C) of approximately -0.031. This means that a buffer prepared at room temperature may not be suitable for use at 0°C to 4°C, as the pH can shift significantly. Additionally, Tris Hydrochloride buffers readily absorb CO2 from the air, requiring strict sealing to prevent pH shifts. Finally, Tris buffers can interfere with certain pH electrodes, necessitating the use of electrodes compatible with Tris solutions. Despite these limitations, Tris Hydrochloride remains a popular choice for many buffering applications due to its versatility and compatibility with biological systems. 2
Reference
1. Tris hydrochloride. Sigma Aldrich. CAS Number: 1185-53-1.
2. 2-Amino-2-(hydroxymethyl)propane-1,3-diol hydrochloride. National Center for Biotechnology Information. 2024; PubChem Compound Summary for CID 93573.
Related articles And Qustion
See also
Lastest Price from TRIS hydrochloride manufacturers

US $0.00/G/KG2025-04-23
- CAS:
- 1185-53-1
- Min. Order:
- 1G/KG
- Purity:
- 99%
- Supply Ability:
- G/KG

US $0.00/Kg/Drum2025-04-21
- CAS:
- 1185-53-1
- Min. Order:
- 1KG
- Purity:
- 99%-101%
- Supply Ability:
- 1000KGS