Trimethoxymethane: applications in organic synthesis
General Description
Trimethoxymethane, also known as trimethyl orthoformate, is an organic compound with the formula HC(OCH3)3. It is a colorless liquid and the simplest orthoester. It is used as a reagent in organic synthesis for the formation of methyl ethers. It plays a crucial role in various reactions such as radical methylation of (hetero)aryl chlorides, enantioselective reactions, light-driven cross-coupling reactions, and the synthesis of allyl alcohol. Overall, trimethoxymethane proves to be a valuable reagent in these synthetic transformations, offering efficient and practical alternatives for various organic synthesis applications.

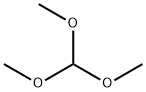
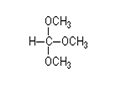
Figure 1. Trimethoxymethane
Applications in organic synthesis
Radical methylation of (hetero)aryl chlorides
Trimethoxymethane plays a crucial role as a methyl source in the radical methylation of (hetero)aryl chlorides. This innovative method utilizes nickel/photoredox catalysis to enable the methylation reaction under mild conditions. Traditionally, the methylation of organohalides requires harsh reaction conditions or reagents, but this approach offers a more efficient and practical alternative. In this methodology, trimethoxymethane, which is commonly used as a laboratory solvent, acts as a convenient source of methyl radicals. The process involves the generation of a tertiary radical via chlorine-mediated hydrogen atom transfer. Subsequently, trimethoxymethane undergoes β-scission, releasing a methyl radical that can selectively methylate (hetero)aryl chlorides and acyl chlorides. One notable advantage of this method is its broad functional group compatibility, allowing for the methylation of diverse substrates. It can be applied in both early and late stage modifications, providing flexibility in synthetic strategies. The use of nickel/photoredox catalysis and trimethoxymethane simplifies the methylation process by offering milder reaction conditions and avoiding the need for more aggressive reagents. Overall, this radical-based approach utilizing trimethoxymethane as a methyl source demonstrates significant potential for the efficient and selective methylation of (hetero)aryl chlorides and acyl chlorides. 1
Enantioselective reaction
Trimethoxymethane plays a crucial role in the reported direct and highly enantioselective reaction involving N-azidoacetyl-1,3-thiazolidine-2-thione. This transformation is catalyzed by Tol-BINAPNiCl2 in the presence of TESOTf and 2,6-lutidine. The reaction allows for the conversion of N-azidoacetyl-1,3-thiazolidine-2-thione into enantiomerically pure 2-azido-3,3-dimethoxypropanamides in high yields. The heterocyclic scaffold of the starting material can be easily removed by adding various amines. Trimethoxymethane serves as a critical methyl source in this enantioselective reaction, enabling the synthesis of enantiomerically pure 2-azido-3,3-dimethoxypropanamides and facilitating the efficient access to lacosamide through a novel C-C bond-forming process. Its use in combination with specific catalysts and reagents demonstrates the utility of trimethoxymethane in complex synthetic transformations. 2
Light-driven cross-coupling reaction
Trimethoxymethane plays a crucial role in the development of light-driven cross-coupling reactions in synthetic organic chemistry. Specifically, it is used as a methyl radical source for C(sp3)-H functionalization reactions. In these reactions, a photoredox catalyst absorbs light and generates an electronically excited charge-transfer state. This excited state can undergo electron or energy transfer with both the substrate and a nickel catalyst. The hypothesis was proposed that photoinduced activation of the nickel catalyst itself could contribute to cross-coupling reactions. The photolysis of a Ni(III) aryl chloride species, formed by single-electron oxidation of a Ni(II) intermediate, allows for the generation of chlorine atoms. Combined with the ability of Ni(II) to accept alkyl radicals, this enables the mediation of hydrogen atom transfer (HAT) with C(sp3)-H bonds. Trimethoxymethane serves as a source of methyl radicals via β-scission from a tertiary radical generated through chlorine-mediated HAT. 3
Synthesis of allyl alcohol
Trimethoxymethane has been employed in the synthesis of allyl alcohol through a series of reactions. Initially, triglycerides were reacted with trimethoxymethane in the presence of camphorsulfonic acid, resulting in the formation of fatty acid methyl esters in high yield. However, this reaction condition did not lead to the formation of the desired protected glycerol. Instead, a separate reaction was carried out using very weak acidic conditions, utilizing catalytic amounts of pyridinium para-toluenesulfonate. This allowed for the formation of orthoesters. To obtain allyl alcohol, the orthoesters were subjected to thermolysis at 270°C, leading to efficient conversion and good yield of the desired product. Overall, trimethoxymethane plays a crucial role in these synthetic transformations, facilitating the synthesis of allyl alcohol through the conversion of triglycerides to FAMEs and the subsequent formation and thermolysis of orthoesters. These reactions offer valuable pathways for the synthesis of allyl alcohol and demonstrate the versatility of trimethoxymethane in organic synthesis. 4
Reference
1. Kariofillis SK, Shields BJ, Tekle-Smith MA, Zacuto MJ, Doyle AG. Nickel/Photoredox-Catalyzed Methylation of (Hetero)aryl Chlorides Using Trimethoxymethane as a Methyl Radical Source. J Am Chem Soc, 2020, 142(16):7683-7689.
2. Teloxa SF, Kennington SCD, Camats M, et al. Direct, Enantioselective, and Nickel(II) Catalyzed Reactions of N-Azidoacetyl Thioimides with Trimethoxymethane: A New Combined Methodology for the Rapid Synthesis of Lacosamide and Derivatives. Chemistry, 2020, 26(50):11540-11548.
3. Kariofillis SK, Doyle AG. Synthetic and Mechanistic Implications of Chlorine Photoelimination in Nickel/Photoredox C(sp3)-H Cross-Coupling. Acc Chem Res, 2021, 54(4):988-1000.
4. Wormann M, Maier ME. Synthesis of allyl alcohol as a method to valorise glycerol from the biodiesel production. RSC Adv, 2019, 9(27):15314-15317.
Related articles And Qustion
See also
Lastest Price from Trimethoxymethane manufacturers

US $0.00-0.00/kg2025-04-21
- CAS:
- 149-73-5
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons
US $0.00/Kg/Drum2025-04-21
- CAS:
- 149-73-5
- Min. Order:
- 200KG
- Purity:
- 99.4
- Supply Ability:
- 1000kg