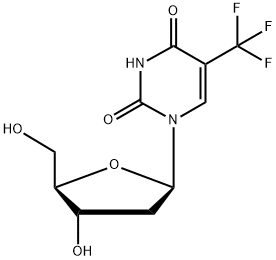
Trifluorothymidine/Trifluoridine
Trifluorothymidine, also known as trifluoridine, is a fluorinated pyrimidine nucleoside analog of thymidine and related to idoxuridine. It differs from thymidine in having three fluorine atoms in the place of three hydrogen atoms in the methyl group. It is distributed by Monarch Pharmaceuticals (Bristol, TN, USA) under3 the brand name Viroptics and by other generic pharmaceutical companies. It is available as a sterile ophthalmic solution that contains 1% trifluoridine in an aqueous solution with acetic acid and sodium acetate (buffers), sodium chloride, and thimerosal 0.001% (added as a preservative). The pH range is 5.5–6.0 and osmolality is approximately 283 mOsm. The chemical name is 2u-deoxy-5-(trifluoromethyl) uridine.
MECHANISM OF DRUG ACTION
Trifluorothymidine, like other nucleoside analogs, is a prodrug which must be phosphorylated to the triphosphate intracellulary before it is active. Trifluorothymidine is monophosphorylated by a cellular thymidine kinase, and then further converted to the triphosphate likewise by cellular enzymes. In this respect, it differs from idoxuridine, aciclovir and penciclovir/famciclovir, all of which are monophosphorylated efficiently only in herpesvirusinfected cells by the virus-specified thymidine kinase. Trifluorothymidine also inhibits CMV, which lacks thymidine kinase, thereby confirming that phosphorylation of trifluorothymidine is wholly dependent on host cell kinases.
Trifluorothymidine monophosphate is an inhibitor of thymidylate synthetase, resulting in depletion of intracellular thymidine nucleotides. This slows viral and host cell DNA syntheses. In addition, trifluorothymidine specifically inhibits viral replication through inhibiting viral DNA polymerases by two separate mechanisms. As a thymidine analog, trifluoridine competitively inhibits the incorporation of the natural nucleotide, thymidine triphosphate, into the growing DNA strand. However, like aciclovir triphosphate, trifluorothymidine is also incorporated into the growing DNA chain, and the DNA with incorporated trifluorothymidine binds irreversibly to the polymerase enzyme, irreversibly inhibiting further DNA synthesis. Herpesvirus DNA polymerases have a higher affinity for trifluoridine triphosphate than the DNA polymerases of mammalian cells, resulting in the preferential incorporation of the analog into viral DNA as opposed to host cell (e.g. human) DNA.
PHARMACOKINETICS
The half-life of trifluorothymidine in the vitreous following intravitreal injection (200 mg) in rabbits was 3.15 hours, and the vitreous concentration remained above the IC50 for CMV for about 30 hours.
Penetration of trifluorothymidine into the eye when applied to the cornea was not enhanced by the use of collagen shields with intact epithelium, whereas in the corneas with abnormal epithelium, drug penetration was higher, but variable. Topically administered trifluorothymidine readily penetrates the cornea, and penetration may be increased in the presence of reduced corneal integrity or local infection. A 2-fold increase in the penetration of trifluorothymidine was observed in the absence of corneal epithelium (United States Pharmacopeia Drug Information, 2005).
Systemic absorption of trifluorothymidine after topical administration through the eye appears to be negligible. The major metabolite determined by in vitro perfusion studies, 5-carboxy-2u-deoxyuridine, is not detected in the aqueous humor or in plasma following topical administration . Trifluorothymidine does not cross the blood–brain barrier. It has been administered topically to the eye concurrently with a number of ophthalmic preparations, including chloramphenicol, dexamethasane, prednisolone, hydrocortisone, atropine sulfate, scopolamine hydrobromide, homatropine hydrobromide, pilocarpine, and epinephrine hydrochloride without adverse interaction.
You may like
See also
Lastest Price from Trifluridine manufacturers

US $0.00/g2025-04-21
- CAS:
- 70-00-8
- Min. Order:
- 1g
- Purity:
- 98%min
- Supply Ability:
- 1000g
US $0.00-0.00/g2025-04-18
- CAS:
- 70-00-8
- Min. Order:
- 10g
- Purity:
- 99%
- Supply Ability:
- 200