Trifluoromethyl(trimethylsilane): A Versatile Reagent in Organic Synthesis
General Description
Trifluoromethyl(trimethylsilane) (TFMTMS) is a useful chemical reagent that contains a trifluoromethyl group and a trimethylsilyl group. It is commonly used as a source of the trifluoromethyl group in various chemical reactions, including in the pharmaceutical industry for the synthesis of drugs. TFMTMS is a topic of interest in chemistry due to its unique properties and versatility in organic reactions. TFMTMS can act as both a Lewis acid and a nucleophile, making it a useful reagent in a wide range of chemical transformations. Its trifluoromethyl group also imparts unique properties to molecules, such as increased stability and lipophilicity, which are desirable in drug design. In addition to its use in pharmaceutical synthesis, TFMTMS has numerous applications in diverse fields like agrochemicals, materials science, and polymers. The ability to selectively introduce trifluoromethyl groups into molecules using TFMTMS has significant implications for chemical synthesis in various industries. As such, research on the potential uses of TFMTMS continues to be an active area of interest and significance in contemporary chemistry.

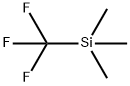
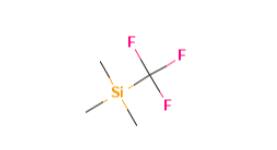
Figure 1. Trifluoromethyl(trimethylsilane)
Properties of TFMTMS
TFMTMS has a chemical formula of C4H9F3Si and a molecular weight of 162.2 g/mol. It is a clear colorless liquid at room temperature with a boiling point of 54-56°C and a melting point of -120°C. The structure of TFMTMS consists of a trifluoromethyl group (-CF3) bonded to a trimethylsilyl group (-Si(CH3)3). The trifluoromethyl group contains a carbon atom triple-bonded to three fluorine atoms, while the trimethylsilyl group contains a silicon atom covalently bonded to three methyl groups. This combination of functional groups gives TFMTMS its unique properties as a versatile reagent for a wide range of chemical transformations. In recent years, there has been increased interest in the development of new reagents and methods for introducing trifluoromethyl groups into organic molecules due to the unique properties they impart in drug discovery and other fields. TFMTMS remains a popular choice in many applications due to its stability and ease of handling in the laboratory. 1
Applications
TFMTMS is a versatile reagent that has diverse applications in various industries such as pharmaceuticals, agrochemicals and materials science. 2 Some of the key applications of TFMTMS in different industries are as follows:
Pharmaceuticals industry
TFMTMS is widely used in the synthesis of drug molecules due to its ability to introduce a trifluoromethyl group (-CF3) into organic molecules. This trifluoromethyl group enhances the biological activity of drug candidates by increasing their lipophilicity, metabolic stability, and receptor affinity. TFMTMS is commonly used in the synthesis of drugs such as antiviral agents, anti-inflammatory agents, anticancer agents, and antidepressants. 3
Agrochemicals industry
TFMTMS is also used in agrochemical synthesis for the development of new pesticides, herbicides, and fungicides with improved activity and selectivity. The introduction of a trifluoromethyl group in agrochemicals can enhance their bioavailability and potency, leading to better crop yields. 4
Materials science
TFMTMS is employed in the production of specialty chemicals and polymers that have unique physical and chemical properties. It is used as a building block in the synthesis of materials such as fluorinated elastomers, fluorine-containing polymers, and coatings for surface protection. 5
Limitations and Challenges
Toxicity
TFMTMS is classified as toxic and requires careful handling and safety protocols to minimize human exposure. Prolonged or repeated exposure can lead to various health problems such as skin, eye, and respiratory irritation. 7
Cost of production
The production and purification methods of TFMTMS can be complex, which adds to the overall cost-effectiveness of its use. This may limit its application in some industries. 1
Environmental impact
Like all chemically active compounds, proper disposal of TFMTMS and its by-products requires attention to environmental regulations. Discharging unused components into the environment can cause potential ecological impacts. 7
Reference
1. Prakash GK, Yudin AK. Perfluoroalkylation with Organosilicon Reagents. Chem Rev, 1997,97(3):757-786.
2. Liebman, J. F., Greenberg, A., Dolbier, W. R., Jr., Eds. Fluorine Containing Molecules: Structure, ReactiVity, Synthesis, and Applications, VCH: New York, 1988.
3. Mu?ller, K. Faeh, C. Diederich, F. Fluorine in Pharmaceuticals: Looking beyond Intuition. Science, 2007, 317:1881?1886.
4. Chu L, Qing FL. Oxidative trifluoromethylation and trifluoromethylthiolation reactions using (trifluoromethyl)trimethylsilane as a nucleophilic CF3 source. Acc Chem Res, 2014, 47(5):1513-1522.
5. Koguchi R, Jankova K, Tanaka M. Fluorine-containing bio-inert polymers: Roles of intermediate water. Acta Biomater, 2022, 138:34-56.
6. Sartori Tamburlin I, Roux E, Feuillée M, et al. Toxicological safety assessment of essential oils used as food supplements to establish safe oral recommended doses. Food Chem Toxicol, 2021, 157:112603.
7. Liu X, Xu C, Wang M, Liu Q. Trifluoromethyltrimethylsilane: nucleophilic trifluoromethylation and beyond. Chem Rev, 2015, 115(2):683-730.
Related articles And Qustion
See also
Lastest Price from (Trifluoromethyl)trimethylsilane manufacturers

US $0.00/KG2025-04-15
- CAS:
- 81290-20-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg
US $0.00-0.00/KG2025-04-04
- CAS:
- 81290-20-2
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1Ton