Trifluoroacetic acid: Physicochemical property, Uses and NMR challenge
Physicochemical property of Trifluoroacetic acid
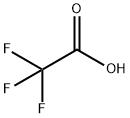
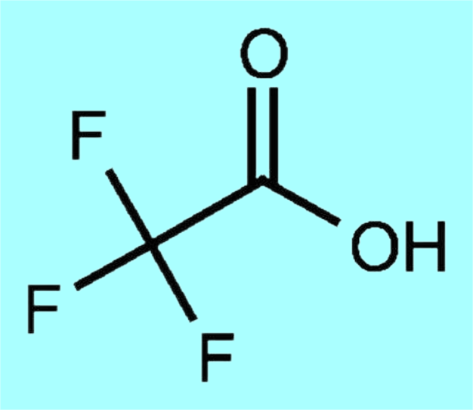
Trifluoroacetic acid (TFA) is an organofluorine compound with the chemical formula CF3CO2H and the structural formula shown below. It is a colourless liquid with a vinegar-like odour. Density is about 1.49 g/cm³, melting point -15.4 °C (lit.), boiling point = 72 ℃, TFA is an analogue of acetic acid (AcOH), due to the high electronegativity of fluorine atoms in TFA and its electron-absorbing effect, its acidity is much stronger than that of AcOH. pKa of TFA is 0.23, and pKa of AcOH is 4.76.
Uses of Trifluoroacetic acid
Trifluoroacetic acid (TFA) is widely used as a solvent, catalyst and reagent in organic synthesis. TFA has the characteristics of low cost, high acidity, easy elimination, high solubility in water and organic solvents, and it is the catalyst of choice for a wide range of reactions such as rearrangement, deprotection of functional groups, oxidation, reduction, condensation, hydroaromatisation, and trifluoromethylation. At low concentrations, TFA is used as an ion-pairing agent in liquid chromatography (HPLC) of organic compounds (especially peptides and small proteins.) TFA is a versatile solvent for nuclear magnetic resonance spectroscopy (for materials stabilised in acids). It is also used as a calibrant in mass spectrometry. In addition, TFA is used as an effective dispersion medium for cellulose nanocrystals and for the preparation of cellulose dextrins.
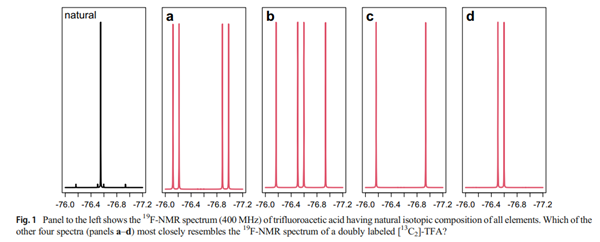
Trifluoroacetic acid NMR challenge
Trifluoroacetic acid is often used as
an ion pairing agent in liquid chromatography to separate peptides and small proteins. Like with the 1
H-NMR
spectrum of ethane, the “textbook” description of its
19F-NMR spectrum consists of a single peak near −
76 ppm relative to CFCl3. This challenge invites you
to consider the TFA wherein both carbon atoms are
carbon-13.
Which of the spectra shown in Fig. 1 most closely resembles the 19F-NMR spectrum of TFA with doubly labeled carbon-13 present?
References:
[1] MARIE-PIER THIBEAULT; Juris M. Trifluoroacetic acid NMR challenge[J]. Analytical and Bioanalytical Chemistry, 2021. DOI:10.1007/s00216-020-02867-3.
[2] R.E. WING; S. N F. Use of trifluoroacetic acid to prepare cellodextrins[J]. Carbohydrate Polymers, 1984. DOI:10.1016/0144-8617(84)90048-1.
[3] JIAHUI WEI M. M A J Lara. Trifluoroacetic Acid as an Effective Dispersing Medium for Cellulose Nanocrystals[J]. SSRN Electronic Journal, 2022. DOI:10.2139/ssrn.4263612.
Related articles And Qustion
See also
Lastest Price from Trifluoroacetic acid manufacturers

US $10.00/KG2025-04-21
- CAS:
- 76-05-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $0.00-0.00/kg2025-04-21
- CAS:
- 76-05-1
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons