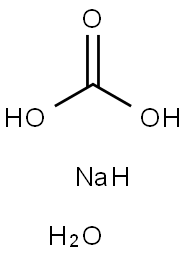
Transformation between sodium carbonate monohydrate and sodium carbonate
Chemical Properties
Sodium carbonate monohydrate (Na2CO3 H2O, CAS registry No. 5968-11-6) is also called soda ash monohydrate, soda monohydrate, which is a white powder. It is stable, but incompatible with strong acids, aluminium, fluorine, lithium, phosphorus oxides.
Sodium carbonate, commonly known as soda ash, is one of the fundamental chemical compounds used in major chemical industries such as using as a source of Na2O in the glass manufacture, in the production of various sodium chemicals for water treatment, paper production, iron desulphurization, and many other uses.
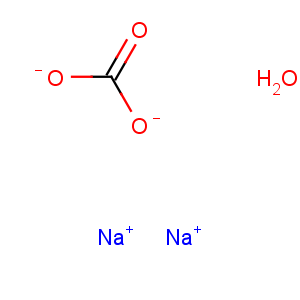
Transformation between sodium carbonate monohydrate and sodium carbonate
It is widely produced in the world commercially by the Solvay process or by refining natural soda ash ores. The latter method is used in countries such as the United States, where ore is available. Soda ash produced from the above methods is available in various grades depending on the physical properties of the crystals such as bulk density and crystal size distribution. Sodium carbonate monohydrate can be obtained from the aqueous solution of the sodium carbonate by atmospheric evaporative crystallization. By this process, the heavy grade (which has a larger average crystal size and higher bulk density) is basically processed from the light grade, resulting in good flow ability and low dust levels to meet customer demand. The feasibility of the process is based on the stability of the two solid phases. The conversion of sodium carbonate to sodium carbonate monohydrate and the subsequent growth of monohydrate crystals have been investigated by carrying out in situ measurements of particle size distribution by means of the focused beam reflectance method (FBRM)[1]. The conversion of sodium carbonate to sodium carbonate monohydrate is found to be a solution-mediated transformation.
Supersaturation nucleation-growth mechanism
The formation of monohydrate crystals is explained by a supersaturation nucleation-growth mechanism. Growth rate calculations show that monohydrate crystals grow at higher rates at lower aqueous phase temperatures, while induction period measurements suggest that sodium carbonate monohydrate crystals nucleate more rapidly at lower temperatures. Both effects can be described by allowing that during the process the solution is saturated with respect to the dissolving sodium carbonate and supersaturated with respect to the growing sodium carbonate monohydrate. As temperature decreases, the supersaturation increases.
Vice versa, the sodium carbonate monohydrate can be converted to anhydrous sodium carbonate by calcining, i.e. by removing the crystal water through heating of the monohydrate to temperatures of 120 oC or higher. For example, the drying and dehydration of the monohydrate is carried out more frequently in steam-tube rotary dryers where the crystals are dehydrated into anhydrous soda ash at 150 oC. As another example, microwave (MW) field with silicon carbide (SiC) as an indirect heating medium can also be used for the dehydration of sodium carbonate monohydrate[2].
References
[1]. Shaikh, A. A.; Salman, A. D.; McNamara, S.; Littlewood, G.; Ramsay, F.; Hounslow, M. J., In situ observation of the conversion of sodium carbonate to sodium carbonate monohydrate in aqueous suspension. Industrial & Engineering Chemistry Research 2005, 44 (26), 9921-9930.
[2]. Seyrankaya, A.; Ozalp, B., Dehydration of sodium carbonate monohydrate with indirect microwave heating. Thermochim. Acta 2006, 448 (1), 31-36.
You may like
Related articles And Qustion
See also
Lastest Price from SODIUM CARBONATE, MONOHYDRATE manufacturers

US $3.00-9.00/KG2025-06-20
- CAS:
- 5968-11-6
- Min. Order:
- 0.01KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available

US $25.00/ASSAYS2025-04-21
- CAS:
- 5968-11-6
- Min. Order:
- 100ASSAYS
- Purity:
- 99.5%
- Supply Ability:
- 100 mt