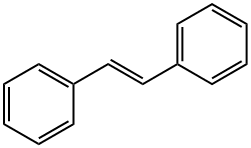
Trans-stilbene--Ecological Information
trans-stilbene, also called (E)-Stilbene or trans-1,2-Diphenylethylene is an organic compound represented by the condensed structural formula C6H5CH=CHC6H5.Trans-stilbene appears as off-white crystals with the melting point of 122-124°C. Here, we introduced some ecological information related to this compound [1].
1. Environmental Fate/Exposure Summary
trans-1,2-Diphenylethylene's production and use in the manufacture of dyes and optical bleaches, and as phosphors and scintillators may result in its release to the environment. If released to air, a vapor pressure of 8.81X10-4 mm Hg at 25 °C indicates trans-1,2-diphenylethylene will exist solely as a vapor in the ambient atmosphere. Vapor-phase trans-1,2-diphenylethylene will be degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals and ozone molecules. The half-life for the reaction in air with hydroxyl radicals is estimated to be 6 hours. The half-life for the reaction in air with ozone is estimated to be 1 hour. If released to soil, trans-1,2-diphenylethylene is expected to have no mobility based upon an estimated Koc of 9,850. Volatilization from moist soil surfaces may be an important fate process based upon an estimated Henry's Law constant of 7.2X10-4 atm-cu m/mole; however, adsorption to soil may attenuate this process.
trans-1,2-Diphenylethylene is not expected to volatilize from dry soil surfaces based upon its vapor pressure. If released into water, trans-1,2-diphenylethylene is expected to adsorb to suspended solids and sediment based upon its estimated Koc. Volatilization from water surfaces may be an important fate process based upon this compound's estimated Henry's Law constant. Estimated volatilization half-lives for a model river and model lake are 2 and 140 hours, respectively. However, volatilization from water surfaces is expected to be attenuated by adsorption to suspended solids and sediment. The estimated volatilation half-life from a model pond is 90 days if adsorption is considered. An estimated BCF of 1,000 suggests the potential for bioconcentration in aquatic organisms is very high. Occupational exposure to trans-1,2-diphenylethylene may occur through inhalation of dust particles and dermal contact with this compound at workplaces where it is produced or used. Limited monitoring data indicate that non-occupational exposure can occur from the ingestion of contaminated drinking water. (SRC)
2. Artificial Pollution Sources
trans-1,2-Diphenylethylene's production and use in the manufacture of dyes and optical bleaches, and as phosphors and scintillators may result in its release to the environment through various waste streams (SRC)[2].
3. Environmental Abiotic Degradation
The rate constant for the vapor-phase reaction of trans-1,2-diphenylethylene with photochemically-produced hydroxyl radicals has been estimated as 6.8X10-11 cu cm/molecule-sec at 25 °C(SRC) using a structure estimation method. This corresponds to an atmospheric half-life of about 6 hours at an atmospheric concentration of 5X10+5 hydroxyl radicals per cu cm. The rate constant for the vapor-phase reaction of trans-1,2-diphenylethylene with ozone molecules has been estimated as 2.5X10-16 cu cm/molecule-sec at 25 °C(SRC) using a structure estimation method. This corresponds to an atmospheric half-life of 1 hour at an atmospheric concentration of 7X10+11 ozone molecules per cu cm. trans-1,2-Diphenylethylene was converted to trans-1,2-diphenylethylene oxide (ratio of conversion was 44%) after 1 hour of irradiation with a xenon lamp (operating through a Pyrex filter) in the presence of nitrogen dioxide in air. trans-1,2-Diphenylethylene is not expected to undergo hydrolysis(SRC) due to the lack of functional groups to hydrolyze.
4. Environmental Bioconcentration
An estimated BCF of 1,000 was calculated for trans-1,2-diphenylethylene(SRC), using a log Kow of 4.81 and a regression-derived equation. According to a classification scheme, this BCF suggests the potential for bioconcentration in aquatic organisms is very high(SRC).
5. Soil Adsorption/Mobility
The Koc of trans-1,2-diphenylethylene is estimated as 9,850(SRC), using a log Kow of 4.81 and a regression-derived equation. According to a classification scheme, this estimated Koc value suggests that trans-1,2-diphenylethylene is expected to be immobile in soil.
6. Environmental Water Concentrations
DRINKING WATER: Trans-1,2-diphenylethylene was detected in drinking water samples from Ottawa in January and February, 1978(1). Trans-1,2-diphenylethylene was quantitatively detected in drinking water in Poplarville, Mississippi in March 1989; Philadelphia, PA in February 1976; and in Ottumwa, Iowa in September 1976(2).
7. Probable Routes of Human Exposure
Occupational exposure to trans-1,2-diphenylethylene may occur through inhalation of dust particles and dermal contact with this compound at workplaces where it is produced or used. Limited monitoring data indicate that non-occupational exposure can occur from the ingestion of contaminated drinking water (SRC) [3].
References
[1] https://pubchem.ncbi.nlm.nih.gov/compound/trans-Stilbene
[2] Lewis RJ Sr; Hawley's Condensed Chemical Dictionary 13th ed NY, NY: John Wiley & Sons Inc p. 1049 (1997)
[3] https://pubchem.ncbi.nlm.nih.gov/compound/trans-Stilbene#section=Artificial-Pollution-Sources
You may like
See also
Lastest Price from TRANS-STILBENE manufacturers
US $8.00-1.00/KG2024-03-29
- CAS:
- 103-30-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available