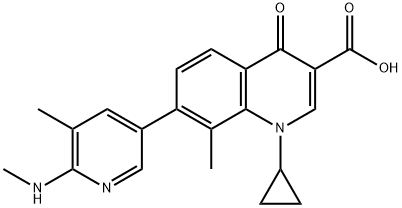
Toxicity of Ozenoxacin
Ozenoxacin is used to treat impetigo (a skin infection caused by bacteria) in adults and children 2 months of age and older. Ozenoxacin is in a class of medications called antibacterials. It works by killing and stopping the growth of bacteria on the skin.
To date, ozenoxacin has been used in trials studying the treatment of impetigo. As of December 11, 2017 the FDA approved Ferrer Internacional S.A.'s Xepi (ozenoxacin 1%) as a topically applied cream indicated for the treatment of impetigo caused by Staphylococccus aureus or Streptococcus pyogenes in adult and pediatric patients 2 months of age and older.
Despite being a common and highly contagious bacerial skin infection that affects millions of children and adults in the United States each year, ozenoxacin cream is a novel, non-fluorinated quinolone that has demonstrated safe and effective therapy in both the adult and pediatric population.
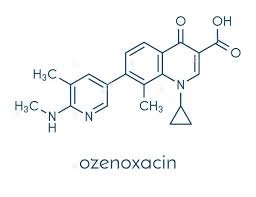
Toxicity
Ozenoxacin cream has the potential to cause rosacea and seborrheic dermatitis when administered topically on a patient. In clinical trials, only one adult patient treated with ozenoxacin cream reported such adverse reactions Label.
Much like other topical antibiotics, the prolonged use of ozenoxacin cream can result in the overgrowth of nonsusceptible bacteria and fungi. If such overgrowths develop into infections during therapy, discontinue use of the ozenoxacin cream and institue appropriate therapy for the infections Label.
Although clinical studies demonstrate negligible systemic absorption after topical administration of ozenoxacin, there are no formal available data on the use of ozenoxacin in pregnant women to inform any professional drug associated risk for use in pregnancy Label.
Although breastfeeding is not expected to result in exposure of the child to ozenoxacin owing to the negligible systemic absorption of ozenoxacin in humans following topical administration, no formal data are available regarding the presence of ozenoxacin in human milk and the effects of ozenoxacin on breasfed infants or on milk production Label.
Clinical experience with ozenoxacin cream has not identified differences in responses between elderly and younger patients Label.The safety and effectiveness of ozenoxacin cream in pediatric patients 2 months and older is similar to that of adults, but the safety and effectiveness of ozenoxacin in pediatric patients younger than 2 months of age has not been formally establlished Label.
Long-term studies in animals to evaluate carcinogenic potential have not been conducted with ozenoxacin Label. Ozenoxacin demonstrated no genotoxicity when evaluated in vitro for gene mutation and/or chromosomal effects in the Ames test, mouse lymphoma cell assay, or when evaluated in vivo in a rat micronucleus test with demonstrated systemic exposure Label.
Oral doses of ozenoxacin did not affect mating and fertility in male and female rats treated up to 500 mg/kg/day (about 8500 and 16,000 times respectively, the maximum human plasma concentration seen with dermal application of ozenoxacin 1% cream) Label.
You may like
Related articles And Qustion
Lastest Price from Ozenoxacin manufacturers

US $0.00-0.00/kg2025-04-21
- CAS:
- 245765-41-7
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 900kg
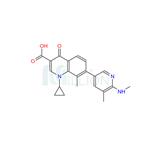
US $1.00-0.50/KG2025-04-21
- CAS:
- 245765-41-7
- Min. Order:
- 2KG
- Purity:
- 99%
- Supply Ability:
- 200KG