Toxicity of Enoxacin
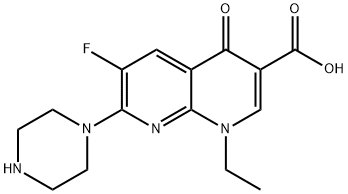
Enoxacin is an early-generation fluoroquinolone with the chemical formula (l-ethyl-6-fluoro-I,4-dihydro-4-oxo-7 piperazinyl)-I,8- naphthyridine-3-carboxylic acid. Overall, the in vitro activity of enoxacin is similar to that of norfloxacin or lomefloxacin but inferior to that of other fluoroquinolones such as ciprofloxacin, ofloxacin, and moxifloxacin. Enoxacin is now rarely used in most regions of the world, but remains available as 200- and 400-mg tablets (Penetrex; Aventis, Guildford, UK) in parts of Europe and Japan.

Bioavailability
Enoxacin is rapidly absorbed after oral administration, but there is individual variation. Absolute oral bioavailability is about 80–90% and is independent of the dose administered. The serum elimination half-life is 3.4–6.8 hours, and protein binding is 35–43%. Gastric acidity is important for maximal enoxacin absorption. Reduction of gastric acidity by agents such as ranitidine reduces the oral bioavailability of enoxacin by about 26%.
Furthermore, antacids such as magnesium–aluminum hydroxide reduce bioavailability by 50% and 73% when given 2 hours and 30 minutes before enoxacin, respectively. The extent of enoxacin absorption and rate of elimination is not altered by co-administration with food; however, a carbohydrate meal delays the time to peak serum concentration by about 0.9 hours. Other foods, including dairy products, do not alter the oral absorption of enoxacin.
Excretion
The kidneys are the main route of excretion for enoxacin, and 54–63% of an administered dose is excreted over a 72-hour period; urinary concentrations are 10- to 100-fold higher than serum levels and remain high for 24 hours. Enoxacin is eliminated predominantly as unchanged drug by the kidney, with peak urine concentrations of 460–690 mg/ml and 1200–1300 mg/ml after 200- and 800-mg doses (either oral or i.v.), respectively. Urine concentrations remain above 20 mg/ml for 24 hours after a 200-mg dose.
Renal clearance of enoxacin is by glomerular filtration (17%78%) and tubular secretion (83%78%). However, it is notable that enoxacin, like some other fluoroquinolones including ciprofloxacin, ofloxacin, and pefloxacin, is less active in urine with MICs increased 8- to 64-fold compared with on standard culture media, possibly because of the presence of higher concentrations of magnesium ions or to the lower pH found in urine. Enoxacin does undergo biliary excretion and levels in bile after a single dose of 400 mg are 17.7 mg/ml. After 7 days of receiving enoxacin, fecal drug concentrations were 100–500 mg/g. Enoxacin is metabolized at the piperazinyl ring to form oxo-, amino-, formyl-, and acetyl-compounds. The major metabolite is the oxo-form, which is found in the serum in concentrations about one-tenth that of the parent compound. In the urine, it accounts for about 15% of the amount of parent enoxacin recovered. The oxo-form has microbiological activity of about one-tenth that of the parent compound. The other metabolites are not found in serum and only as traces in urine.
Toxicity
Enoxacin-associated side-effects are similar to those encountered with other fluoroquinolones and include rashes, photosensitivity reactions, headache, dizziness, convulsions, hallucinations, depression, nausea, anorexia, hypoglycemia, eosinophilia, and leukopenia. Possible acute cholestatic or hepatitic injury associated with enoxacin has also been reported.
See also
Lastest Price from Enoxacin manufacturers

US $0.00-0.00/KG2025-05-28
- CAS:
- 74011-58-8
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 10000KGS

US $0.00/KG2025-04-24
- CAS:
- 74011-58-8
- Min. Order:
- 1KG
- Purity:
- 0.99
- Supply Ability:
- 1000KG