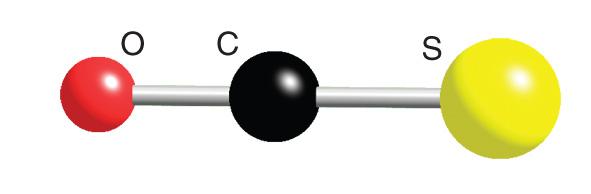
Toxicity of Carbonyl sulfide
Carbonyl sulfide (COS) is a colorless, odorless (when pure)
relatively stable gas with a boiling point of -50°C.
There are limited commercial uses of COS. It is produced
only in small quantities and used for small-scale experimental
purposes and as an intermediate in the synthesis of organic
sulfur compounds, thiocarbamate herbicides, and alkyl
carbonates.
Uses
Pesticide manufacturers are believed to be the largest
users of COS. Similar to CS2, research conducted by the Stored
Grain Research Laboratory at the Australia’s Commonwealth
Scientific and Industrial Research Organisation (CSIRO) has
shown COS to be an effective soil and grain fumigant for
controlling insects on crops such as wheat, barley, oats, and peas,
although it is not currently approved for this commercial use.
The use of COS as a fumigant for durable commodities and structures was patented worldwide in 1992 by CSIRO Australia. COS has the potential to replace methyl bromide, being phased out due to its ozone depletion properties, in several of its applications for durable commodities and also to be used as an alternative to phosphine when there is a significant problem with insect resistance.
Environmental Fate
Most of the releases of COS to the environment are to air,
where it is believed to have a long residence time. Its half-life
in the atmosphere is estimated to be approximately 2 years.
It may be degraded in the atmosphere via a reaction with
photochemically produced hydroxyl radicals or oxygen,
direct photolysis, and other unknown processes related to the
sulfur cycle. Sulfur dioxide, a greenhouse gas, is ultimately
produced from these reactions. COS is relatively unreactive in
the troposphere, but direct photolysis may occur in the stratosphere. Also, plants and soil microorganisms have been
reported to remove COS directly from the atmosphere. Plants
are not expected to store COS.
COS is extremely mobile in soils. If released to soil, it will
volatilize quickly to the atmosphere (Koc= 88). It has a high
solubility in water and will not readily adsorb to soil particles,
sediment, or suspended organic matter. Therefore, COS is expected
to volatilize rapidly from soil and water or, depending on
volume, concentration, and site-specific characteristics (e.g., soil
type, depth to groundwater, temperature, and humidity), maybe
able to move rapidly through the ground and impact groundwater.
COS may be hydrolyzed in water to form H2S and CO2.
COS is also actively taken up by some plants and converted
to CS2; that is, the atmospheric pathways are reversed,
and soils may act as both a net source and a net sink for COS
depending on the concentration of COS and the characteristics
of the soil. COS is therefore accurately described as
a naturally occurring and widely distributed chemical found
or produced in the air, soils, live and decomposing vegetation,
and food.
Toxicity
Toxicity from exposure to COS is likely the result of its decomposition to CO2 and H2S. H2S inhibits respiration at the cellular level, causing methemoglobinemia, which inhibits the cytochrome oxidase system, causing cytotoxic anoxia. In one study, rats treated with acetazolamide, an inhibitor of carbonic anhydrase, showed lower blood levels of H2S following exposure to COS and exhibited decreased toxicity. H2S is believed to be primarily responsible for many of the reported adverse effects associated with exposure to COS.
COS reacts readily with ammonia and primary amines to
form ammonium thiocarbamate and amine salts of monothiocarbamic
acid, respectively. Reaction with two primary
amines may result in the formation of H2S and linking of the
two amines via a carbonyl group reaction, suggesting considerable
potential for protein cross-linking by COS in vivo, and
this has been proposed as a mechanism to explain occupational
neuropathy observed with CS2, and predicted for COS.