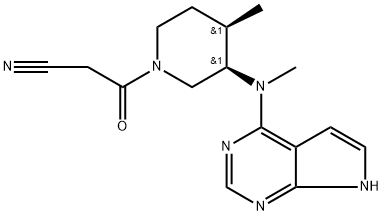
Tofacitinib:a versatile JAK Inhibitor in autoimmune conditions
Introduction
Tofacitinib is a medication that belongs to a class of drugs known as Janus kinase (JAK) inhibitors. It is used to treat certain autoimmune conditions, particularly rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis. Tofacitinib works by inhibiting the activity of Janus kinases, which are enzymes involved in the signaling pathways of the immune system1.Psoriasis and psoriatic arthritis are the main clinical mani festations of psoriatic disease (PsD). Both have complex and heterogeneous phenotypic expression which may represent fundamentally diverse disease sub-types. Although initially thought to be relatively benign disorders, more recent work has demonstrated the destructive and progressive nature of the arthritis and significant associated cardiovascular co morbidities with increased mortality. At the same time, advances in treatment, and in particular with biological drugs, have provided effective tools with which to control the many facets of the disease.
Application
Psoriasis and psoriatic arthritis (PsA) are inflammatory immune-mediated conditions which can cause considerable disability and reduced quality of life. Management can be complex as clinical heterogeneity may lead to different treatment pathways. Tofacitinib is a novel, oral Janus Kinase (JAK) inhibitor with proven efficacy in rheumatoid arthritis. Areas covered: This review analyzes recent studies of tofacitinib in psoriatic disease treatment. The relevant literature was identified using clinicaltrials.gov, PubMed, and Google Scholar. Tofacitinib efficacy was demonstrated in PsA by the OPAL Broaden and OPAL Beyond phase-III studies, and received FDA and EMA approval. Tofacitinib was superior to placebo for the treatment of moderate-to-severe plaque psoriasis in the OPT Pivotal 1 and 2, OPT Retreatment studies, but FDA approval was declined for this indication based on issues of clinical efficacy and long-term safety. Expert commentary: Tofacitinib is an important oral drug for the treatment of PsA. However, the long-term safety data require further evaluation. Tofacitinib and other JAK inhibitors show potential to broaden the treatment options in PsA and other inflammatory conditions2. In addition, also applicable to other diseases, for example: 1. Rheumatoid Arthritis: Tofacitinib is approved for the treatment of moderate to severe rheumatoid arthritis in adults who have not responded well to methotrexate. 2.Psoriatic Arthritis: It is also used for the treatment of active psoriatic arthritis. 3.Ulcerative Colitis: Tofacitinib is indicated for the treatment of moderate to severe ulcerative colitis in adults who have not responded well to other medications. Mechanism of Action:Tofacitinib inhibits Janus kinases (JAKs), specifically JAK1 and JAK3. By doing so, it interferes with the signaling pathways that contribute to the inflammatory and immune responses seen in autoimmune diseases.
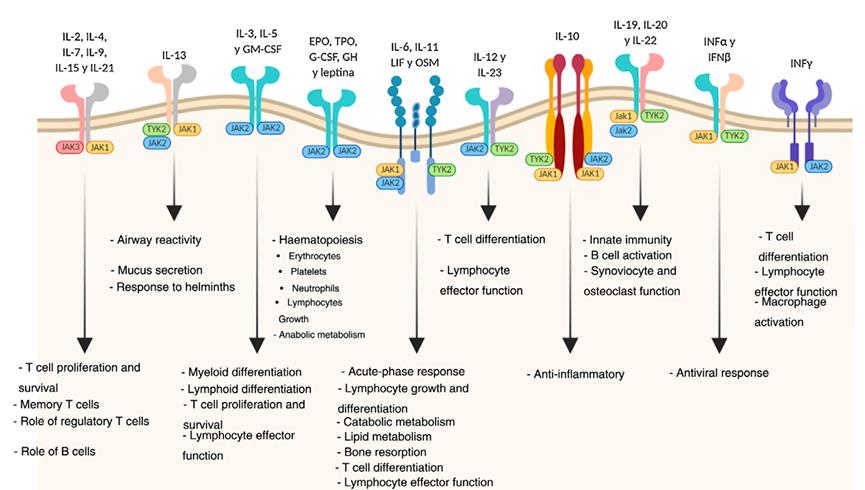
Figure 1 Biological functions of the JAK pathways
Synthesis
The synthesizing process of JAK3 inhibitor tofacitinib citrate was improved based on the analysis of the methods previously published. Using 2, 4-dichloro 7H-pyrrolo [2, 3-d] pyrimidine and (3R, 4R)-1-benzyl-N, 4 dimethylpiperidin-3-amine dihydrochloride as starting materials, tofacitinib citrate was obtained through four steps of nucleophilic substitution, catalytic transfer hydrogenation, cyanide acetylation and citrate salt, and its crystal form was consistent with the original research. After optimization, the yield was better than those reported in literature, and the mild reaction conditions were suitable for industrial production.
Safety
Tofacitinib is a potent, selective JAK inhibitor that preferentially inhibits Janus kinase 1 and JAK3. In the EU, oral tofacitinib 5 mg twice daily is indicated for the treatment of moderate to severe active rheumatoid arthritis in adult patients who have responded inadequately to, or who are intolerant of, one or more DMARDs. Several clinical studies of ≤ 24 months' duration showed that tofacitinib monotherapy (as first- or second-line treatment) and combination therapy with a conventional synthetic DMARD; as second- or third-line treatment) was effective in reducing signs and symptoms of disease and improving health-related quality of life, with benefits sustained during long-term therapy (≤ 96 months). Tofacitinib monotherapy inhibited progression of structural damage in methotrexate-naïve patients during ≤ 24 months' treatment, with beneficial effects also seen in patients receiving tofacitinib plus methotrexate as second-line therapy for 12 months. Tofacitinib was generally well tolerated during ≤ 114 months' treatment, with most adverse events of mild or moderate severity. The tolerability profile of tofacitinib was generally similar to that of biological DMARD, with infections and infestations the most common adverse events in tofacitinib recipients. However, the incidence of herpes zoster was higher with tofacitinib than in the general rheumatoid arthritis population, although infections were clinically manageable. When added to background methotrexate, tofacitinib was noninferior to adalimumab in terms of efficacy, and both combination therapies had generally similar tolerability profiles. Although additional comparative studies are needed to more definitively position tofacitinib relative to bDMARDs and other targeted synthetic DMARDs, current evidence indicates that oral tofacitinib is a useful option for the treatment of patients with rheumatoid arthritis4.
The use of Janus kinase (JAK) inhibitors is a new approach in the therapy of inflammatory diseases with immune base. It is a small synthetic molecule administered orally, with a fast bioavailability and elimination rate, predictable pharmacokinetics and lack of immunogenicity, which are convenient characteristics for both efficacy and safety3. Administration: Tofacitinib is typically taken orally in the form of tablets. Monitoring: Patients taking tofacitinib may require regular monitoring of blood counts, liver function, and lipid levels. Adverse Effects: Common side effects include upper respiratory tract infections, headaches, and high blood pressure. Serious side effects may include an increased risk of serious infections, certain cancers, and liver problems. Contraindications: Tofacitinib is generally not recommended for use in individuals with a history of serious infections, certain gastrointestinal perforations, or lymphoma. Pregnancy and Breastfeeding: Tofacitinib may have potential risks during pregnancy and breastfeeding, and its use should be carefully considered in these situations.
Reference
1. Sandborn WJ, Su C, Sands BE, D'Haens GR, Vermeire S, Schreiber S, Danese S, Feagan BG, Reinisch W, Niezychowski W, Friedman G, Lawendy N, Yu D, Woodworth D, Mukherjee A, Zhang H, Healey P, Panés J; OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2017 May 4;376(18):1723-1736.
2. Berekmeri A, Mahmood F, Wittmann M, Helliwell P. Tofacitinib for the treatment of psoriasis and psoriatic arthritis. Expert Rev Clin Immunol. 2018 Sep;14(9):719-730.
3. López-Sanromán A, Esplugues JV, Domènech E. Pharmacology and safety of tofacitinib in ulcerative colitis. Gastroenterol Hepatol. 2021 Jan;44(1):39-48. English, Spanish.
4. Dhillon S. Tofacitinib: A Review in Rheumatoid Arthritis. Drugs. 2017 Dec;77(18):1987-2001.
You may like
Related articles And Qustion
See also
Lastest Price from Tofacitinib manufacturers

US $1.00/KG2025-04-21
- CAS:
- 477600-75-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $150.00/kg2025-04-21
- CAS:
- 477600-75-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 500kg