Thymine:a pyrimidine nucleobase
Introduction
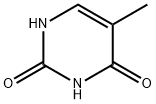
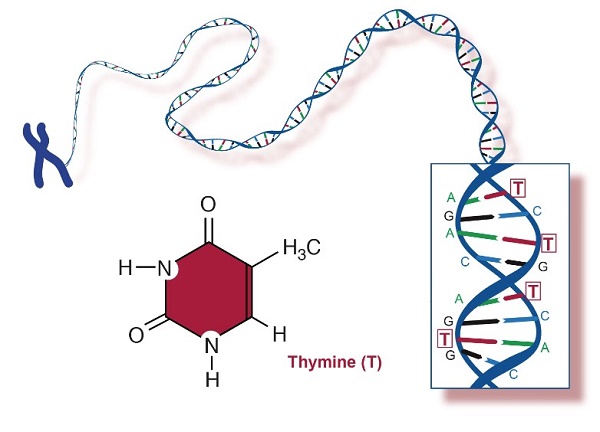
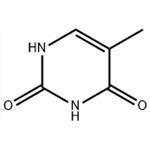
Thymine is a pyrimidine nucleobase that is uracil in which the hydrogen at position 5 is replaced by a methyl group. It has a role as a human metabolite, an Escherichia coli metabolite and a mouse metabolite. It is a pyrimidine nucleobase and a pyrimidone. It is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). Thymine is a natural product found in Synechocystis, Tectitethya, and other organisms with data available.Thymine is one of the four nucleotide bases that make up DNA (deoxyribonucleic acid), the genetic material in living organisms. The other three bases are adenine (A), cytosine (C), and guanine (G). Thymine is represented by the letter T.
Application
In DNA, thymine always pairs with adenine through hydrogen bonding, forming a base pair. This complementary base pairing is a crucial aspect of the double-stranded structure of DNA. The sequence of these base pairs encodes genetic information, and the specific order of the bases along the DNA molecule determines the genetic code. In RNA (ribonucleic acid), which is another type of nucleic acid involved in protein synthesis, thymine is replaced by uracil (U). RNA uses adenine, cytosine, guanine, and uracil as its four bases. Thymine's primary application lies within its integral role in the structure of DNA, forming complementary base pairs with adenine to create the genetic code that instructs cellular functions. Understanding the sequence of thymine within DNA enables researchers to decipher and manipulate genetic information, contributing to advancements in fields such as genetics, biotechnology, and medicine. Techniques like polymerase chain reaction (PCR), genetic engineering, and gene sequencing heavily rely on the specific pairing properties of thymine to replicate, modify, or analyze DNA sequences. Thymine's significance extends to diagnostics, drug development, and genetic research, making it a foundational element in numerous applications that deepen our understanding of life sciences and facilitate innovations in various scientific domains.
Two new structures of the quadruplex d(TGGGGT)4 obtained by single crystal X-ray diffraction. In one of them a thymine tetrad is found. Thus the yeast telomere sequences d(TG1-3) might be able to form continuous quadruplex structures, involving both guanine and thymine tetrads. Our study also shows substantial differences in the arrangement of thymines when compared with previous studies. We find five different types of organization: (i) groove binding with hydrogen bonds to guanines from a neighbour quadruplex; (ii) partially ordered groove binding, without any hydrogen bond; (iii) stacked thymine triads, formed at the 3'ends of the quadruplexes; (iv) a thymine tetrad between two guanine tetrads. Thymines are stabilized in pairs by single hydrogen bonds. A central sodium ion interacts with two thymines and contributes to the tetrad structure. (v) Completely disordered thymines which do not show any clear location in the crystal. The tetrads are stabilized by either Na+ or Tl+ ions3.
Synthesis
DNA can be sequenced by a chemical procedure that breaks a terminally labeled DNA molecule partially at each repetition of a base. The lengths of the labeled fragments then identify the positions of that base. We describe reactions that cleave DNA preferentially at guanines, at adenines, at cytosines and thymines equally, and at cytosines alone. When the products of these four reactions are resolved by size, by electrophoresis on a polyacrylamide gel, the DNA sequence can be read from the pattern of radioactive bands. The technique will permit sequencing of at least 100 bases from the point of labeling1.
The wild-type (top) and salvage (bottom) pathways for Thymine synthesis(Figure 1):Wild-type species of bacteria are unable to pump thymine into the cells and to incorporate it into DNA. Thyauxotrophs lack thymidylate synthetase activity and, hence, cannot produce thymidine nucleotides by the natural route (top panel), while they gained the ability to incorporate thymine into DNA by condensing it with deoxyribose-1-phosphate (dRib-1-P) to thymidine, a reaction catalyzed by thymidine phosphorylase (bottom). The negligible pool of dRib-1-P in thy+ cells that limits this condensation increases substantially in size in thyA mutants, since they accumulate U-dRib-P, which is degraded into dRib-1-P through U-dRib. Addition of a nucleoside such as 2′-deoxyguanosine (dG) similarly results in a wild-type pool size of dRib-1-P (6). Blocking further catabolism of the latter by deoB or deoC mutation results in a still higher pool size and, hence, lower thymine concentrations (2 rather than 20 μg ml−1) are sufficient to support growth of thyA strains carrying at least one of these mutations; such double or triple mutants are “thymine-low-requirers.” At least two additional mutations affect the regulation of thymidine phosphorylase to convert the cell to a “super-low requirer” (can grow on concentrations as low as 0.2 μg ml−1). Mutants lacking thymidine phosphorylase cannot utilize thymine, and those lacking thymidine kinase fail to incorporate thymidine as well2.
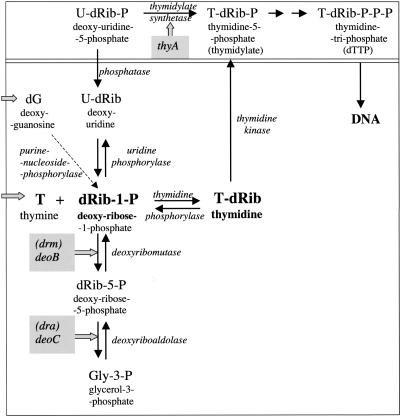
Figure 1 The wild-type (top) and salvage (bottom) pathways for Thymine synthesis
Safety
Thymine is safe as a natural part of DNA in living organisms. However, isolated thymine supplements are not commonly used, and their safety is not well-established. It's generally safer to obtain nutrients from a balanced diet rather than through isolated supplements. If you have concerns, consult with a healthcare professional.
Reference
1. Maxam AM, Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560-4.
2. Carter CS, Kenkel WM, MacLean EL, Wilson SR, Perkeybile AM, Yee JR, Ferris CF, Nazarloo HP, Porges SW, Davis JM, Connelly JJ, Kingsbury MA. Is Oxytocin "Nature's Medicine"? Pharmacol Rev. 2020 Oct;72(4):829-861.
3. Cáceres C, Wright G, Gouyette C, Parkinson G, Subirana JA. A thymine tetrad in d(TGGGGT) quadruplexes stabilized with Tl+/Na+ ions. Nucleic Acids Res. 2004 Feb 11;32(3):1097-102.
You may like
Related articles And Qustion
See also
Lastest Price from Thymine manufacturers

US $0.00/kg2025-08-26
- CAS:
- 65-71-4
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons
US $0.00-0.00/Kg2025-04-21
- CAS:
- 65-71-4
- Min. Order:
- 1Kg
- Purity:
- 98%
- Supply Ability:
- 20Ton