The Uses and indications Cetuximab
Introduction
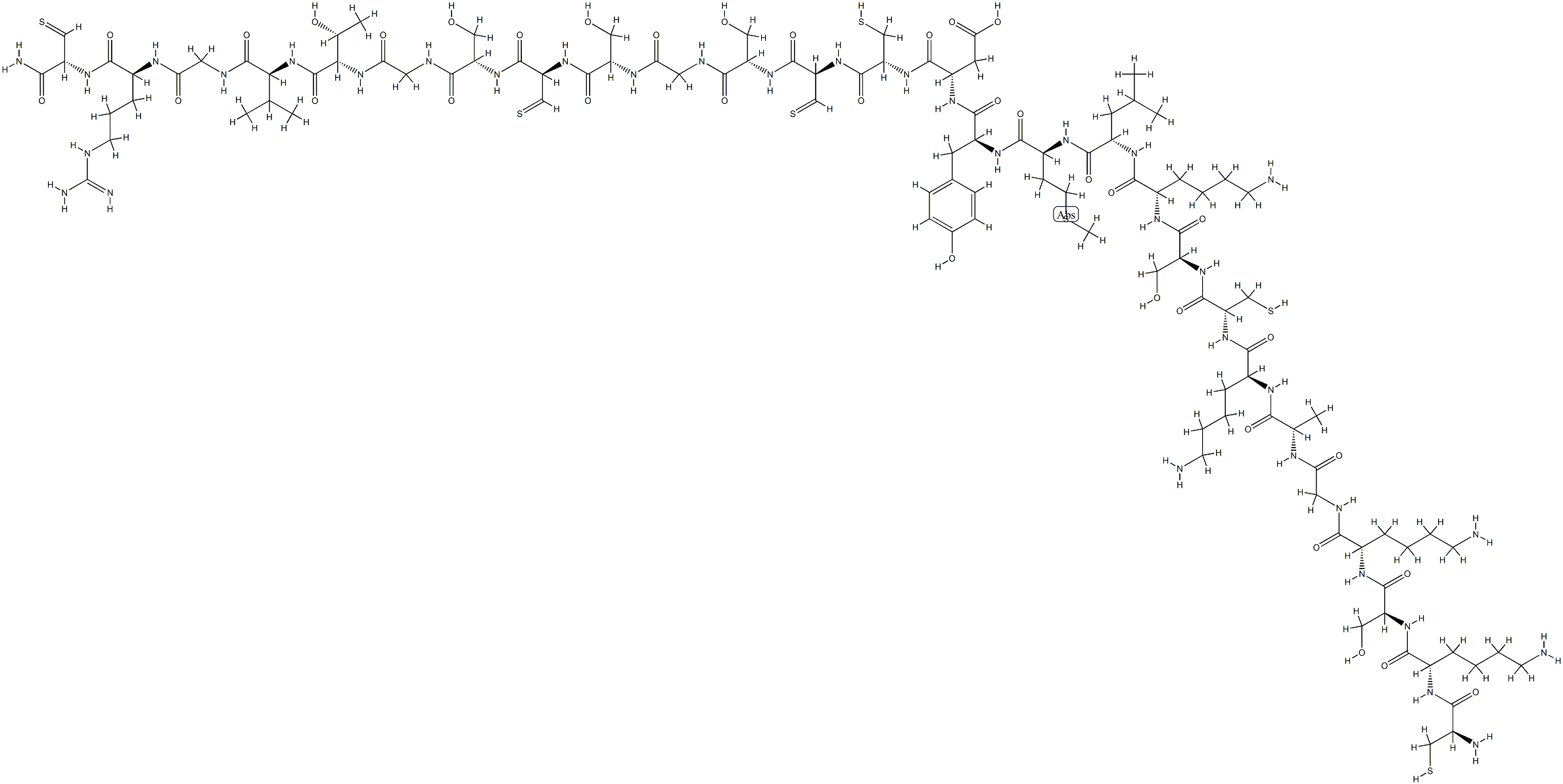
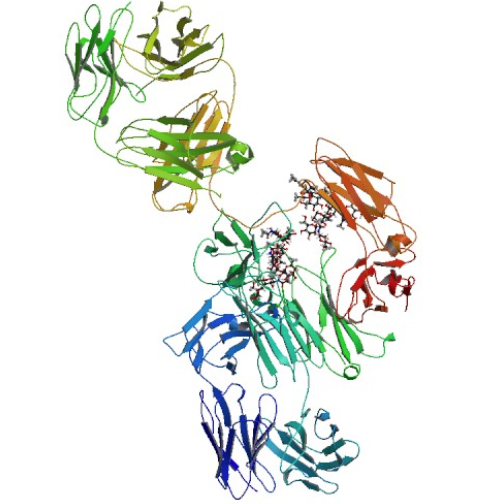
Cetuximab is a chimeric human-murine monoclonal antibody against EGFR. Cetuximab has been approved since 2006 for the treatment of SCCHN, in combination with radiation therapy as the initial treatment of locally or regionally advanced tumors and as a single agent for patients with platinum-resistant cancers such as recurrent or metastatic SCCHN. This drug has also been approved since 2004 for the treatment of EGFR-expressing metastatic colorectal cancer as a single agent or in combination with irinotecan for patients with chemotherapy-refractory cancers[1].
Uses and indications
FDA-Approved Uses
Colorectal cancer, metastatic, KRAS wild-type (without mutation) - Cetuximab improves both overall survival and progression-free survival and preserves quality-of-life measures for patients with colorectal cancer where other treatments have failed. There is a limitation of use. Patients with colorectal tumor-bearing mutated K-ras did not benefit from cetuximab, whereas patients with tumor-bearing wild-type K-ras benefited from cetuximab. If KRAS mutation in either codon 12 or 13 is detected, then patients with metastatic colorectal carcinoma should not receive anti-EGFR antibody therapy.
Head and neck cancer (squamous cell) - Treatment for locoregionally advanced head and neck cancer with concomitant high-dose radiotherapy plus cetuximab demonstrates improvement of locoregional control and lowers mortality without increasing the common toxic effects associated with radiotherapy to the head and neck. Cetuximab plus platinum-fluorouracil chemotherapy improved overall survival when given as first-line treatment for patients with recurrent or metastatic squamous-cell carcinoma of the head and neck.
Non-FDA-Approved Uses
Colorectal cancer, advanced, biweekly administration
Non-small cell lung cancer (NSCLC), EGFR-expressing, advanced
Squamous cell skin cancer, unresectable
Side effect
Cutaneous side effects are the most common adverse reactions occurring during epidermal growth factor receptor inhibitors (EGFRI) therapy. Papulopustular rash (acne-like rash) develops in 80- 86% of patients receiving cetuximab, while xerosis, eczema, fissures, teleangiectasiae, hyperpigmentations, and nail and hair changes occur less frequently. The mechanism underlying these skin changes has yet to be established and understood. The epidermal growth factor receptor inhibition alters cell growth and differentiation, leading to impaired stratum corneum and cell apoptosis[2].
References
[1] W Bou-Assaly, S Mukherji. “Cetuximab (erbitux).” American Journal of Neuroradiology 31 4 (2010): 626–7.
[2] Daška Štulhofer Buzina. “Adverse Reaction to Cetuximab, an Epidermal Growth Factor Receptor Inhibitor.” Acta Dermatovenerologica Croatica 24 1 (2016): 70–2.
Related articles And Qustion
See also
Lastest Price from Cetuximab manufacturers

US $0.00/g2025-01-13
- CAS:
- 205923-56-4
- Min. Order:
- 1g
- Purity:
- More Than 99%
- Supply Ability:
- 100kg/Month

US $1.10/g2021-07-03
- CAS:
- 205923-56-4
- Min. Order:
- 1g
- Purity:
- 99.9%
- Supply Ability:
- 100 Tons Min