Cetuximab: A Beacon in Targeted Cancer Therapy and Precision Medicine
Cetuximab is an epidermal growth factor receptor (EGFR) inhibitor with the following FDA-approved indications: colorectal cancer, metastatic, KRAS wild-type (without mutation), and head and neck cancer (squamous cell).

Mechanism of Action
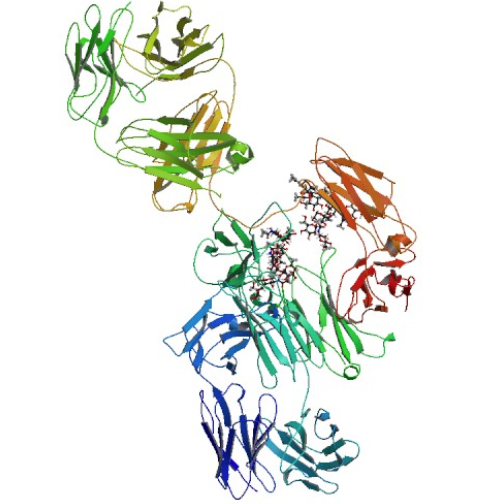
Cetuximab is a recombinant chimeric human/mouse IgG1 monoclonal antibody which binds to epidermal growth factor receptor (EGFR) and competitively inhibits the binding of epidermal growth factor (EGF) and other ligands. EGFR is a member of the ErbB family of receptors. When inactive, EGFR is a monomer, but when bound by epidermal growth factor or transforming growth factor-alpha (TGF-alpha), it forms homodimers or heterodimers with another member of the ErbB family of receptors. Dimerization activates the intracellular tyrosine kinase region of EGFR, resulting in autophosphorylation and initiating a cascade of intracellular events. The EGFR signaling pathway regulates cell differentiation, proliferation, migration, angiogenesis, and apoptosis, all of which become deregulated in cancer cells. Cetuximab binds to EGFR with high specificity and a higher affinity than either epidermal growth factor or TGF-alpha, thus blocking the ligand-induced phosphorylation of EGFR. Also, cetuximab enhances the effects of irinotecan and radiotherapy in experimental systems. K-ras, a small G-protein downstream of EGFR and a vital component of the EGFR signaling cascade, can acquire activating mutations in exon 2, thus isolating the pathway from the effect of EGFR and rendering EGFR inhibitors ineffective.
Uses
Cetuximab is an epidermal growth factor receptor (EGFR) inhibitor medication used for the treatment of metastatic colorectal cancer and head and neck cancer. It is a chimeric (mouse/human) monoclonal antibody given by intravenous infusion.
Adverse Effects
Adverse effects include the following based on body systems:
Central nervous system: Fatigue (91%), malaise (73% or less), pain (59%), peripheral sensory neuropathy (45%; grades 3/4: 1%), headache (19% to 38%), insomnia (27%), confusion (18%), chills (16% or less), rigors (16%or less), anxiety (14%), depression (14%)
Dermatologic: Desquamation (95%), acneiform eruption (15% to 88%; grades 3/4: 1% to 18%), radiodermatitis (86%), xeroderma (14% to 57%), pruritus (14% to 47%), skin rash (28% to 44%), changes in nails (31%), acne vulgaris (14% to 22%), paronychia (20%), palmar-plantar erythrodysesthesia (19%), skin fissure (19%), alopecia (12%)
Endocrine & metabolic: Weight loss (15% to 84%), hypomagnesemia (6% to 55%), dehydration (13% to 25%), hypocalcemia (12%), hypokalemia (12%)
Gastrointestinal: Diarrhea (19% to 72%), nausea (49% to 64%), abdominal pain (59%), constipation (53%), vomiting (40%), stomatitis (31% to 32%), anorexia (25% to 30%), dyspepsia (14% to 16%), xerostomia (12%)
Hematologic and oncologic: Neutropenia (49%; grades 3/4: 31%), leukopenia (grades 3/4: 17%)
Hepatic: Increased serum ALT (43%), increased serum AST (38%), increased serum alkaline phosphatase (33%)
Infection: Infection (13% to 44%), infection without neutropenia (38%)
Local: Application site reaction (18%)
Neuromuscular and skeletal: Weakness (73% or less), ostealgia (15%), arthralgia (14%)
Ophthalmic: Conjunctivitis (10% to 18%)
Respiratory: Dyspnea (49%), cough (30%), pharyngitis (26%)
Miscellaneous: Fever (22% to 29%), infusion related reaction (10% to 18%; grades 3/4: 2% to 5%.
You may like
Related articles And Qustion
See also
Lastest Price from Cetuximab manufacturers

US $0.00/g2025-01-13
- CAS:
- 205923-56-4
- Min. Order:
- 1g
- Purity:
- More Than 99%
- Supply Ability:
- 100kg/Month

US $1.10/g2021-07-03
- CAS:
- 205923-56-4
- Min. Order:
- 1g
- Purity:
- 99.9%
- Supply Ability:
- 100 Tons Min